Acid Base & Salt - Class 10th Science
NCERT Exercise solution:2
Question: 7: Why does distilled water not conduct electricity, whereas rain water does?
Answer: If an aqueous solution contains ions, then it conducts electricity otherwise not.
Since distilled water does not contain any salt and consequently does not conduct electricity. On the other hand rain water contains many salts as impurities. These salts dissociate ions in rain water because of which rain water conducts electricity.
This is the cause that distilled water does not conduct electricity whereas rain water conducts.
Question: 8: Why do acids not show acidic behavior in the absence of water?
Answer: An acid shows acidic behavior because of presence of hydrogen ions. Acid dissociates hydrogen ions only in their aqueous solution. This is the cause that acids do not show acidic behavior in the absence of water.
Question: 9: Five solutions A, B, C, D and E when tested with universal indicator showed pH as 4, 1, 11, 7 and 9 respectively. Which solution is
- Neutral?
- Strongly alkaline?
- Strongly acidic?
- Weakly acidic?
- Weakly alkaline?
Arrange the pH in increasing order of hydrogen ion concentration.
Answer: Hydrogen ions concentration increases with decrease in pH value from 7 consequently strength of acid increases with decrease in pH value from 7 to 0.
On the other hand hydroxide ions concentration increases with increase in pH value from 7 consequently strength of acid increases with increase in pH value from 7 to 14.
While neutral solution has pH value equal to 7.
Therefore,
(a) Solution D is neutral having pH value equal to 7
(b) Solution C is strongly alkaline as its pH value is equal to 11
(c) Solution B is strongly acidic as its pH value is equal to 1
(d) Solution A is weakly acidic as its pH value is equal to 4
(e) Solution E is weakly alkaline as its pH value is equal to 9
Arrangement of given pH value in increasing order of hydrogen ion concentration:
11 < 9 < 7 < 4 < 1
Question: 10: Equal lengths of magnesium ribbons are taken in test tubes A and B. Hydrochloric acid (HCl) is added to test tube A, while acetic acid (CH3COOH) is added to test tube B. Amount and concentration taken for both acids are same. In which test tube will the fizzing occur more vigorously and why?
Answer: Hydrochloric acid is stronger acid than acetic acid. Because of this, hydrochloric acid reacts with magnesium ribbon more vigorously than acetic acid.
Thus, in test tube A in which hydrochloric acid is added with magnesium ribbon fizzing occurs more vigorously than test tube B in which acetic acid is added.
This happens because hydrochloric acid liberates hydrogen gas more vigorously than acetic acid.
Question: 11: Fresh milk has a pH of 6. How do you think the pH will change as it turns into curd? Explain your answer.
Answer: Milk and curd both contains lactic acid. Curd has more concentration of lactic acid than milk.
Thus, when milk is turns into curd concentration of hydrogen ions increase since concentration of lactic acid increases. Thus, with increase in concentration of hydrogen ions pH value will decrease when milk turns into curd.
Question: 12: A milkman adds a very small amount of baking soda to fresh milk.
- Why does he shift the pH of the fresh milk from 6 to slightly alkaline?
- Why does this milk take a long time to set as curd?
Answer: Milk starts curdling with increase in concentration of lactic acid produced by bacteria present in milk, i.e. curdling starts with decrease in pH value.
(a) Milkman shifts pH of fresh milk from 6 to slightly alkaline to delay the lactic acid produced by bacteria and hence milk takes more time to automatic curdling by adding a very small amount of baking soda.
(b) Acid present in milk is neutralized by adding of baking soda. That’s why this slightly alkaline milk takes long time to set as curd as production of acid takes more time.
Question: 13: Plaster of Paris should be stored in a moisture proof container. Explain why?
Answer: When plaster of Paris exposed to moisture present in air, it is converted into gypsum by absorbing water. This gypsum after some time starts drying and sets into hard mass.
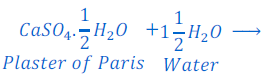
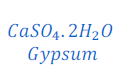
This is the cause that Plaster of Paris should be stored in a moisture proof container to prevent it getting moisture from air.
Question: 14: What is the neutralization reaction? Give two examples.
Answer: Neutralization of an acid after reaction with a base is called neutralization reaction. In other words, when an acid reacts with a base, base neutralizes base after formation of water and respective salt, this is called neutralization reaction.
Example:
(a) When hydrochloric acid reacts with sodium hydroxide (base), it produces sodium chloride and water because of neutralization reaction.
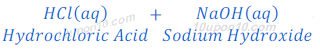
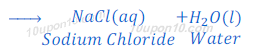
(b) When suplhuric acid reacts with sodium hydroxide, sodium sulphate and water are formed after neutralization reaction.
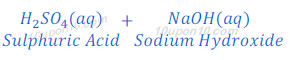
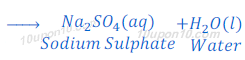
Question: 15: Give two important uses of washing soda and baking soda?
Answer:
Uses of washing soda:
(a) Used as detergent in cleaning of clothes
(b) Used to remove the permanent hardness of water
Uses of baking soda:
(a) In bakery to make batter soft
(b)Use as one of the material in fire extinguisher