Acid Base & Salt - Class 10th Science
NCERT Exemplar Solution:MCQs3
Question: 21. Which of the following is used for dissolution of gold?
- Hydrochloric acid
- Sulphuric acid
- Nitric acid
- Aqua regia
Answer: (d) Aqua regia
Explanation: Aqua regia dissolved gold.
Question: 22. Which of the following is not a mineral acid?
- Hydrochloric acid
- Citric acid
- Sulphuric acid
- Nitric acid
Answer: (b) Citric acid
Explanation: Other acids than citric acid are mineral acids.
Question: 23. Which among the following is not a base?
- NaOH
- KOH
- NH4OH
- C2H5 OH
Answer: (d) C2H5 OH
Explanation: C2H5 OH is ethyl alcohol and not a base.
Question: 24. Which of the following statements is not correct?
- All metal carbonates react with acid to give a salt, water and carbon dioxide
- All metal oxides react with water to give salt and acid
- Some metals react with acids to give salt and hydrogen
- Some non metal oxides react with water to form an acid
Answer: (b) All metal oxides react with water to give salt and acid.
Question: 25. Match the chemical substances given in Column (A) with their appropriate application given in
| Column(A) | Column(B) |
|---|---|
| (A) Bleaching powder | (i) Preparation of glass |
| (B) Baking soda | (ii)Production of H2 and Cl2 |
| (C) Washing soda | (iii)Decolourisation |
| (D) Sodium chloride | (iv) Antacid |
- A–(ii), B–(i), C–(iv), D–(iii)
- A–(iii), B–(ii), C–(iv), D–(i)
- A–(iii), B–(iv), C–(i), D–(ii)
- A–(ii), B–(iv), C–(i), D–(iii)
Answer: (c) A – (iii), B – (iv), C – (i), D – (ii)
Question: 26. Equal volumes of hydrochloric acid and sodium hydroxide solutions of same concentration are mixed and the pH of the resulting solution is checked with a pH paper. What would be the colour obtained? (You may use colour guide given in Figure 2.2
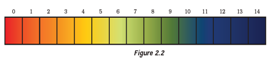
- Red
- Yellow
- Yellowish green
- Blue
Answer: (c) Yellowish green
Explanation: Hydrochloric acid and sodium hydroxide neutralizes each other and pH of the resulting solution will be 7. Thus, option (c) Yellowish green is the correct answer.
Question: 27. Which of the following is(are) true when HCl (g) is passed through water?
- It does not ionise in the solution as it is a covalent compound.
- It ionises in the solution
- It gives both hydrogen and hydroxyl ion in the solution
- It forms hydronium ion in the solution due to the combination of hydrogen ion with water molecule
- (i) only
- (iii) only
- (ii) and (iv)
- (iii) and (iv)
Answer: (c) (ii) and (iv)
Question: 28. Which of the following statements is true for acids?
- Bitter and change red litmus to blue
- Sour and change red litmus to blue
- Sour and change blue litmus to red
- Bitter and change blue litmus to red
Answer: (c) Sour and change blue litmus to red
Question: 29. Which of the following are present in a dilute aqueous solution of hydrochloric acid?
- H3O+ + Cl—
- H3O+ + OH—
- C—– + OH—
- unionised HCl
Answer: (a) H3O+ + Cl—
Question: 30. Identify the correct representation of reaction occurring during chloralkali process
- 2NaCl(l) + 2H2O(l) → 2NaOH(l) + Cl2 (g) + H2 (g)
- 2NaCl(aq) + 2H2O(aq) → 2NaOH(aq) + Cl2 (g) + H2 (g)
- 2NaCl(aq) + 2H2O(l) → 2NaOH(aq) + Cl2 (aq) + H2 (aq)
- 2NaCl (aq) + 2H2O (l) → 2NaOH (aq) + Cl2 (g) + H2 (g)
Answer: (d) 2NaCl (aq) + 2H2O (l) → 2NaOH (aq) + Cl2 (g) + H2 (g)