Metals & Non-metals - Class 10th Science
NCERT Exercise Solution2
Question: 7. Name two metals which will displace hydrogen from dilute acids, and two metals which will not.
Answer:
Zinc and magnesium displace hydrogen from dilute acid.
Copper and silver do not displace hydrogen from dilute acid.
When zinc react with dilute sulphuric acid, it gives zinc sulphate and hydrogen gas.

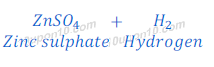
When magnesium reacts with dilute hydrochloric acid, it gives magnesium chloride and hydrogen gas.
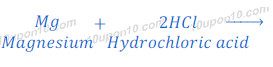

Question: 8. In the electrolytic refining of a metal M, what would you take as the anode, the cathode and the electrolyte?
Answer: In the electrolytic refining of a metal M,
(a) A thick lump of impure metal M is taken as anode
(b) A thin strip of pure metal M is taken as cathode
(c) Water soluble salt of metal M is taken as electrolyte.
Question: 9. Pratyush took sulphur powder on a spatula and heated it. He collected the gas evolved by inverting a test tube over it, as shown in figure below.
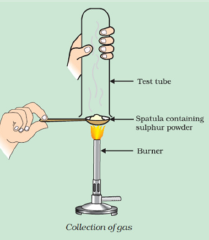
(a) What will be the action of gas on
(i) dry litmus paper?
(ii) moist litmus paper?
(b) Write a balanced chemical equation for the reaction taking place.
Answer: (a) Action of gas on
(i) dry litmus paper: Dry litmus paper do not turns red or blue with acid or base. So there is no effect on dry litmus paper.
(ii) moist litmus paper: Gas evolved is sulphur dioxide. Since, sulphur dioxide is a non-metallic oxide and non-metallic oxides are acidic in nature. Thus, moist blue litmus paper turns red.
(b) Balance chemical equation for the reaction taking place:
When sulphur is burnt or heated in air, it gives sulphur dioxide gas.

Question: 10. State two ways to prevent the rusting of iron.
Answer: Rusting of iron can be prevented by preventing iron comes in contact with moisture present in air. There are many ways to prevent rusting two of them are as follows:
(a) Painting: By painting articles made of iron, rusting can be prevented. Coat of paint protects iron from coming in contact with moisture present in air.
(b) Galvanisation: Galvanisation is the process of depositing a layer of zinc over articles made of iron. A thin layer of zinc over iron metal, prevent it to come in contact with moist air, and rusting is prevented.
Question: 11. What type of oxides are formed when non-metals combine with oxygen?
Answer: When non-metals combine with oxygen, acidic oxides are formed.
Examples:
Carbon is a non-meal. When carbon (coal) is burnt in air, it forms carbon dioxide.
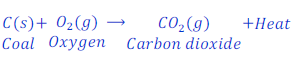
Carbon dioxide is a non-metallic oxide, when carbon dioxide dissolve in water it produces carbonic acid.
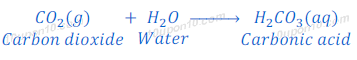
Question: 12. Give reasons
(a) Platinum, gold and silver are used to make jewellery.
Answer: Platinum, gold and silver are used to make jewellery because of following reasons:
(i) These metals are least reactive and hence able to keep their shine for longer time.
(ii) These metals are highly malleable and ductile and hence can be mould easily.
(b) Sodium, potassium and lithium are stored under oil.
Answer: Sodium, potassium and lithium are highly reactive metals. These reacts immediately even with moisture present in air. So, these metals are stored under oil to prevent any reaction and any accident.
(c) Aluminium is a highly reactive metal, yet it is used to make utensils for cooking.
Answer: Aluminium reacts with oxygen present in air and forms layer of aluminium oxide over it. This aluminium oxide prevents further reaction of aluminium with oxygen present in air this makes aluminium corrosion resistant. Apart from this aluminium is a good conductor of heat. These are the cause that aluminium is used to make utensils for cooking instead of it is a highly reactive metal.
(d) Carbonate and sulphide ores are usually converted into oxides during the process of extraction.
Answer: Metal oxides can be easily reduced to respective metals this makes extraction of metals easy using metal oxides. Thus carbonate and sulphide ores are usually converted into oxides during the process of extraction.
Question: 13. You must have seen tarnished copper vessels being cleaned with lemon or tamarind juice. Explain why these sour substances are effective in cleaning the vessels.
Answer: Copper is tarnished because of formation of layer of carbonate over it. Carbonate is basic which can be dissolved or neutralized when reacts with acids. Lemon or tamarind juice contain acid, thus when tamarind or lemon juice is rubbed over tarnished copper vessels, layer of carbonate get dissolved and removed from the surface of copper vessels. After removal of layer of carbonates from surface, copper vessels or other items made of copper get its natural metallic shine. This is the cause that sour substances are effective in cleaning the vessels made of copper.
Question: 14. Differentiate between metal and non-metal on the basis of their chemical properties.
Answer: Difference between metal and non-metal on the basis of their chemical properties:
(i) Metals give hydrogen gas when react with dilute acid while non-metals do not react with dilute acids.
(ii) Metals give basic oxide when react with oxygen while non-metals give acidic oxide.
(iii) Metals form ionic bonds with non-metals while non-metals form covalent bonds when reacts with non-metals.
(iv) Metals reacts with water and gives hydrogen gas while non-metals do not react with water.
Question: 15. A man went door to door posing as a goldsmith. He promised to bring back the glitter of old and dull gold ornaments. An unsuspecting lady gave a set of gold bangles to him which he dipped in a particular solution. The bangles sparkled like new but their weight was reduced drastically. The lady was upset but after a futile argument the man beat a hasty retreat. Can you play the detective to find out the nature of the solution he had used?
Answer: Aquaregia is the mixture of hydrochloric acid and nitric acid in the ratio of 3:1. Aqua-regia is a very strong acid which dissolve gold in it. Gold gets dissolved in aqua-regia can be obtained again. Auqaregia is used to clean gold ornaments.
The man uses auqa-regia as solution which dissolves dirty layer of gold bangles along with some of the layer of gold. Since, outer and tarnished layer of gold bangles get dissolved so bangles starts shining again.
Question: 16. Give reasons why copper is used to make hot water tanks and not steel (an alloy of iron).
Answer: Copper is used to make hot water tanks and not steel because
(i) Copper does not get rust like steel (iron).
(ii) Copper is a noble metal and hence corrosion resistant
(iii) Copper is good conductor of heat and electricity.
(iv) Above reasons make hot water tank made of copper a more durable and suitable than that of steel.