Solutions
Abnormal Molar Masses
Molar masses that are lower or higher than expected values when calculated (generally using colligative properties) are called abnormal molar masses.
Abnormal molar masses depends upon the total number of moles particles either after dissociation or association of solute molecules in solvent or solution.
Dissociation of Solutes:
There are many solutes, such as organic acids, salts and bases, when dissolved in water dissociate into ions.
Example – When one mole of NaCl is dissolved into water, it dissociates into Na + and Cl - . After dissociation, there would be one mole of sodium ions (Na+) and one mole of chloride ions (Cl- ) released in solution. Thus, we get two moles of particles in the solution.
After the dissociation of NaCl or KCl or any other solutes which breaks into ions into water, the boiling point of water increases.
And when molar mass is determined experimentally, it always comes lower than the expected value.
Association of Solutes:
There are many solutes, when dissolved, get associated into the solution, this decreases the number of moles into the solution. This increases the molar mass of the solution than expected value, when determined experimentally, using colligative properties methods.
Example –
When acetic acid (ethanoic acid) is dissolved into benzene, molecules of acetic acid gets associated i.e. dimerize due to hydrogen bondings and number of particles reduced. This happens generally in the solvents having low dielectric constants.
This increases the molar mass than expected value when determined experimentally.
Van’t Hoff Factor:
Van’t Hoff, a Dutch scientist, after many experiments, introduced a factor known as Van’t Hoff factor after keeping in account of the increase or decrease in molar masses than expected because of dissociation or association of solutes.
The Van’t Hoff Factor is denoted by letter ‘i’.
Van’t Hoff defined this factor as follows:
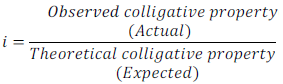
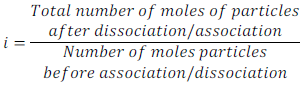
Where abnormal molar mass is determined experimentally and colligative properties is obtained by keeping in mind that solute (non-volatile) neither associated nor dissociated.
In the case of dissociation 
And in the case of association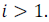
Thus, equations for colligative properties can be modified as follows after inclusion of Van’t Hoff factors.
Relative lowering of vapour pressure of solvent
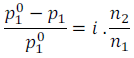
Elevation of boiling point,
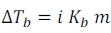
Depression of Freezing point,
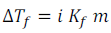
Osmotic pressure of solutions,
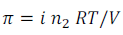
Reference: