Solutions
Solubility of a Gas in a Liquid
Many gases dissolve in water. For example – Oxygen dissolve in water. It is the dissolved oxygen in water which sustains all aquatic life.
Effect of pressure on solubility of gas in liquid
Solubility of a gas in liquid increases with increase in pressure.
Explanation:
Since gas is highly compressible, thus increase in pressure increases the number of gaseous particle per volume.
The solubility of gas keeps increasing with increasing in pressure until a new dynamic equilibrium reached.
Henry’s Law:
Henry’s Law states that at constant temperature the solubility of as gas in a liquid is directly proportional to the pressure of the gas.
If mole fraction is used as a measure of solubility in a liquid then Henry’s Law can be stated as: –
The mole fraction of gas in the solution is proportional to the partial pressure of the gas over the solution.
In other words Henry’s Law can be stated as –
The partial pressure of the gas in vapour phase (p) is directly proportional to the mole fraction of the gas (x) in the solution.
Thus,
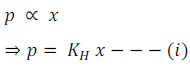
Where , KH is called Henry’s Law constant.
It is clear from the above Henry’s Law equation that
(i) Solubility of a gas in liquid increases with increase in pressure and vice verse. That is solubility of gas in liquid decreases with decrease in pressure.
(ii) The solubility of a gas in liquid decreases with increase in Henry’s Law constant (KH), at a given pressure.
This means at a given pressure higher the value of KH (Henry’s Law constant), the lower is the solubility of the gas in a liquid and vice versa.
Effect of temperature on solubility of gas in a liquid:
Solubility of a gas in a liquid decreases with increase in temperature.
Dissolution of gas in liquid is considered similar to condensation and heat is evolved in this process. Since dissolution of gas in liquid is an exothermic process the increase in temperature resulting in decrease in solubility of gas in liquid.
This is the cause that aquatic animals are more comfortable in cold water rather than in warm water.
Application of Henry’s Law:
(1) Since solubility of gas in liquid increases with increase in pressure, hence to increase the solubility of CO2 in soft drinks and soda water, the bottle is sealed under high pressure.
(2) Scuba divers have to face the problems of bends. When they dive and go deeper in water, pressure gradually increases resulting in increase in the solubility of atmospheric gases in blood.
Bends is a medical condition which blocks the blood capillaries due to formation of bubbles of nitrogen in blood. Bends is very painful and dangerous to life.
When scuba divers come towards the surface of water they have to face bends. Hence scuba diver use a tank filled with air diluted with helium (11.7% helium, 56.2% nitrogen and 32.1% oxygen) to avoid bends as well as toxic effects of high concentration of nitrogen in the blood because of increase in pressure underwater and decreasing pressure towards the water surface.
(3) People, who live at high altitudes and climbers (mountaineers) have to face a problem called anoxia. Anoxia is also a medical condition.
The partial pressure of oxygen in atmosphere decreases with high altitude. Decrease in partial pressure of oxygen leads to low concentration of oxygen in blood and tissues. Because of low concentration of oxygen people feel short of breathe and become weak and unable to think clearly.
To avoid the condition of anoxia mountaineers use oxygen cylinder while climbing to high altitude.
Reference: