Solutions
Vapour pressure of liquid solution
When a liquid solution is kept in a closed container, some of the molecules of liquid from its surface starts evaporating and start accumulating in the space above the surface of the liquid.
Molecules of liquid in the vapour phase move randomly and strike the liquid surface and get condensed. The process keeps on until equilibrium between evaporation and condensation reached.
The pressure exerted by molecules of vapour phase present in the vacant space of the container over the liquid surface is called vapour pressure of liquid.
Vapour pressure of liquid-liquid solution:
Figure of vapour pressure
Raoult’s Law
Francois Marte Raoult, a French chemist, establish the quantitative relation between partial pressure and mole fraction of components of liquid-liquid solution, which is known as Raoult’s Law.
Raoult’s Law states that for a solution of volatile liquids the partial vapour pressure of each component in the solution is directly proportional to its mole fraction.
Let us consider a binary solution of two volatile liquids 1 and 2.
Let partial pressure of component (1) of the solution 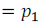
And partial pressure of component (2) of the solution 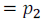
Let the total vapour pressure at equilibrium 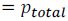
Let mole fraction of component (1) 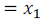
Let mole fraction of component (2) 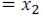
Therefore, according to Raoult’s Law
For component (1)
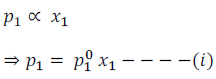
Similarly, for component (2)
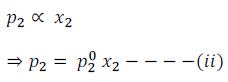
Where 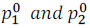 are the vapour pressures of pure component (1) and (2) respectively.
are the vapour pressures of pure component (1) and (2) respectively.
Now, According to Datlton’s Law of Partial Pressure, the total vapour pressure over the solution phase in the closed container is equal to the sum of partial pressure of components of the solution.
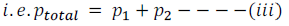
Thus, after substituting the values of equations (i) and (ii) in equation (iii), we get
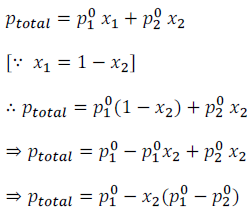
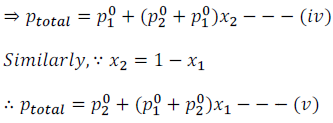
From the above equations (iv) and (v) it can be said:
(1) Total vapour pressure over the solution is related to the molar fraction of any one of the component of solution.
(2) Total vapour pressure over the solution varies linearly with the mole fraction of one of the components of solution.
(3) Total vapour pressure over the solution decreases or increases with the increase of the molar fraction of component depending on the vapour pressure of the pure component.
Graph of partial pressure p1 or p2 versus mole fractions x1 and x2 is shown in the figure.
Figure of graph
From the graph it is evident that-
(1) Line (I) and (II) are linear and passes through the points when x1 and x2 are equal unity respectively.
(2) Graph of ptotal verses x2 also comes linear.
(3) Depending upon the vapour pressures of the pure components, total vapour pressure over the solution may increase or decrease with increase in the mole fraction of a component.
The composition of vapour phase in equilibrium:
This is determined by the partial pressure of the components.
Using Dalton’s Law of Partial Pressure,
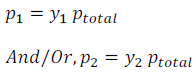
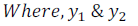 are the mole fractions of the component 1 and 2 respectively.
are the mole fractions of the component 1 and 2 respectively.
Reference: