Solutions
Ideal and Non-ideal solution
Ideal Solutions
Solutions which obey Raoult’s Law over the entire range of concentration are known as Ideal Solutions.
Ideal solutions have two important properties:
(a) Enthalpy of mixing of the pure components to form solution is equal to zero.
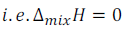
This means that no heat is absorbed or evolved when components (solute and solvent) are mixed.
(b) Volume of mixing is equal to zero.
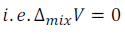
This means that volume of the solution will be equal to the sum of the volume of components (solute and solvent).
Behaviour of Ideal Solutions at molecular level
Let consider two components in an Ideal Solution, A(solute) and B(Solvent). In pure component the intermolecular interactions will be of A-A and B-B types, while in a binary solution intermolecular interaction will be A-B types also in addition to A-A and B-B types.
If the intermolecular attractive forces between A-A and B-B are nearly equal to that of A-B, then such solution is called ideal solution.
Practically an ideal solution does not exists. But there are some solutions which show nearly ideal behavior or can be called nearly ideal solution.
Example –
Solution of n-hexane and n-heptane, solution of chloroethane and bromoethane, solution of benzene and toluene, etc. are nearly ideal solutions.
Non-ideal solution
Solutions which do not obey the Raoults law over the entire range of concentration, are called non-ideal solutions.
The vapour pressure of non-ideal solutions is either higher or lower than the ideal, i.e. predicted by Raoult’s Law.
If the vapour pressure of solution is higher than the predicted, then it shows positive deviation and if lower than the predicted, then it shows negative deviation from Raoult’s Law.
Reference: