Solutions
Vapour pressure of solutions of solid in liquid
When a solid is dissolved in a liquid the vapour pressure of the solution is because of the molecules of solvent only in vapour phase.
In the case of pure liquid (solvent) the entire surface of the liquid is occupied by liquid molecules only, but when a solid solute is dissolved in the liquid, some of the surface of solution is occupied by the molecules of dissolved solute, resulting in the decrease in the concentration of molecules of solvent (liquid) at surface.
This resulting in reduction of number of molecules of solvent escaping from the surface which reduces the vapour pressure of solution compare to the vapour pressure of pure liquid.
The decrease in vapour pressure of solvent (pure liquid) depends upon the quantity of solid solute dissolved in the solution and not on the nature of solute.
Example – At same temperature decrease in vapour pressure of water by adding 0.1 mol of sucrose to 1kg of water and decrease in vapour pressure of water by adding 0.1 mol of urea to the same quantity of water is nearly equal.
In the solid (non-volatile) – liquid solution:
Let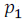 is the vapour pressure of solvent
is the vapour pressure of solvent
Let 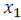 is the mole fraction of solvent.
is the mole fraction of solvent.
Let 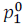 is the vapour pressure of pure solvent.
is the vapour pressure of pure solvent.
Therefore, according to Raoult’s Law
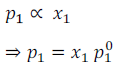
Here,  is the vapour pressure of pure solvent and is equal to the proportionality constant.
is the vapour pressure of pure solvent and is equal to the proportionality constant.
Graph
Reference: