Solutions
Elevation of Boiling Point
Boiling Point
The temperature at which the vapour pressure is equal to the atmospheric pressure is called the boiling point of a liquid.
Vapour pressure of a liquid increases with increase in temperature.
We know that boiling point of a solution becomes higher than that of the boiling point of pure solvent in which the solution is prepared.
The elevation of boiling point depends upon the number of solute molecules irrespective of their nature as similar to lowering of vapour pressure.
Let 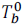 be the boiling point of pure solvent.
be the boiling point of pure solvent.
And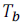 is the boiling point of solution.
is the boiling point of solution.
Therefore, the increase in boiling point 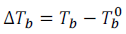 is known as the elevation of boiling point.
is known as the elevation of boiling point.
From several experiment, it has been established that for dilute solutions the elevation of boiling point is directly proportional to the molal concentration of the solute in a solution.
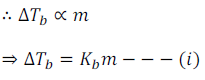
Here, m (molality) is the number of moles of solute dissolved in 1 kg of solvent.
Kb is called the Boiling Point Elevation constant or Molal Elevation Constant or Ebullioscopic Constant.
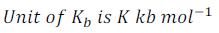
Let, w2 gram of solute of molar mass M2 is dissolved in w1 gm of solvent.
Therefore, molality (m) of the solution can be expressed as
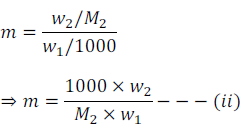
By putting the value of ‘m’ from equation (ii) in equation (i) we get
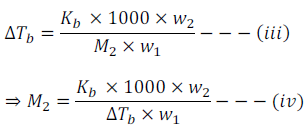
Thus, using above equation Molar mass can be calculated by knowing other quantities.
Reference: