Acid Base & Salt - Class 10th Science
Sodium Hydroxide
Sodium hydroxide is also known as caustic soda or Iye. Sodium hydroxide comes in the form of pellets, flakes, granules, and solution in market. Sodium hydroxide is a strong base and caustic in nature.
Production of sodium hydroxide from sodium chloride
CHLOR-ALKALI PROCESS:
Sodium hydroxide is obtained by the Chlor-Alkali Process. In this Chlor stands for chlorine and alkali stands for sodium hydroxide, that’s why this is called chlor-alkali process.
In chlor-alkali process electric current is passed through the brine (aqueous solution of sodium chloride). After passing the electric current through brine, sodium hydroxide solution is formed near cathode. In this process chlorine gas is deposited at anode and hydrogen gas is deposited at cathode.
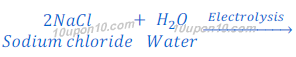
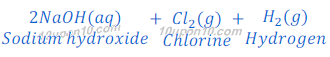
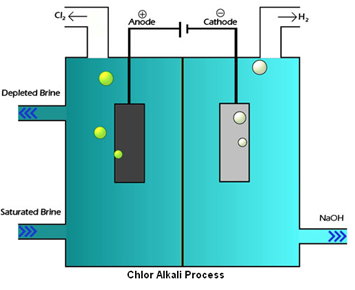
Chlor-alkali process – fig1
https://en.wikipedia.org/wiki/File:Membrane_cell.jpg#filelinksUses of products obtained in CHLOR-ALKALI PROCESS:
Sodium hydroxide:
In making of soaps
In making of detergents
In making of paper
In making of artificial fibres
In de-greasing of metals, etc.
Chlorine:
In water treatement
Mixed with swimining pool water
In making of PVC
In making of disinfectants
In making of CFCs
In making of pesticides
Hydrogen:
In making of fuels
Margarine
In making of ammonia for fertilizers
Chlorine + Hydrogen:
Chlorine and hydrogen together is used in making of hydrochloric acid. Hydrochloric acid is also known as Muriatic Acid and spirits of salt.
Hydrochloric acid is used in cleaning of steel, making of ammonium chloride, in making of medicines and cosmetics, etc.
Chlorine + Sodium hydroxide:
Chlorine and sodium hydroxide together is used in making of bleach for household bleaches and bleaching of fabrics, etc.