Acid Base & Salt - Class 10th Science
NCERT Exemplar Solution:Short Answer2
Question: 38. Salt A commonly used in bakery products on heating gets converted into another salt B which itself is used for removal of hardness of water and a gas C is evolved. The gas C when passed through lime water, turns it milky. Identify A, B and C.
Answer: When baking powder is heated, it produces carbon dioxide, water and sodium carbonate. Sodium carbonate is used for removal of hardness of water.

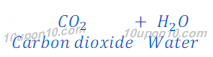
Therefore, given salt A is baking powder (sodium hydrogen carbonate) which is used in bakery products to make batter soft.
Salt B is sodium carbonate. This is used for removal of hardness of water.
Gas C is carbon dioxide, which when passes through lime water, lime water turns milky.
Question: 39. In one of the industrial processes used for manufacture of sodium hydroxide, a gas X is formed as by product. The gas X reacts with lime water to give a compound Y which is used as a bleaching agent in chemical industry. Identify X and Y giving the chemical equation of the reactions involved.
Answer:
In Solvay process, this is an Industrial process, used for manufacture of sodium hydroxide from brine (aqueous solution of sodium chloride).
When electric current is passed through brine, chlorine gas and hydrogen gases are formed along with sodium hydroxide.
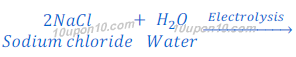
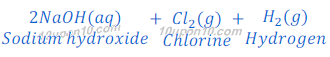
One of the bi-products in this process is chlorine. When chlorine reacts with lime water (calcium hydroxide), calcium oxychloride (bleaching powder) is formed.
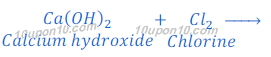
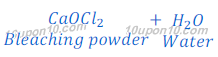
This bleaching powder is used as a bleaching agent in chemical industry.
Thus,
Gas ‘X’ is chlorine
Compound ‘Y’ is calcium oxychloride (bleaching powder)
Question: 40. Fill in the missing data in the following table
| Name of salt | Formula | Salt obtained from | |
|---|---|---|---|
| Base | Acid | ||
| (i)Ammonium chloride | NH4Cl | NH4OH | — |
| (ii)Copper sulphate | — | — | H2SO4 |
| (iii)Sodium chloride | NaCl | NaOH | — |
| (iv)Magnesium nitrate | Mg(NO3 )2 | — | HNO3 |
| (v)Potassium sulphate | K2SO4 | — | — |
| (vi)Calcium nitrate | Ca(NO3 )2 | Ca(OH)2 | — |
Answer:
| Name of salt | Formula | Salt obtained from | |
|---|---|---|---|
| Base | Acid | ||
| (i)Ammonium chloride | NH4Cl | NH4OH | HCl |
| (ii)Copper sulphate | CuSO4 | Cu(OH)2 | H2SO4 |
| (iii)Sodium chloride | NaCl | NaOH | HCl |
| (iv)Magnesium nitrate | Mg(NO3 )2 | Mg(OH)2 | HNO3 |
| (v)Potassium sulphate | K2SO4 | KOH | H2SO4 |
| (vi)Calcium nitrate | Ca(NO3 )2 | Ca(OH)2 | HNO3 |
Question: 41. What are strong and weak acids? In the following list of acids, separate strong acids from weak acids.
Hydrochloric acid, citric acid, acetic acid, nitric acid, formic acid, sulphuric acid.
Answer:
Strong Acid: Acids that ionize completely in their aqueous solution are called strong acids. Most of the mineral acids are strong.
Weak Acid: Acids that do not ionize completely in their aqueous solution are called weak acids. Most of the organic acids are weak.
Hydrochloric acid, Nitric Acid, Sulphuric Acid: Strong Acid
Citric Acid, Acetic Acid, Formic Acid: Weak Acid
Question: 42. When zinc metal is treated with a dilute solution of a strong acid, a gas is evolved, which is utilized in the hydrogenation of oil. Name the gas evolved. Write the chemical equation of the reaction involved and also write a test to detect the gas formed.
Answer: When zinc metal is treated with dilute solution of sulphuric acid or other strong acids, zinc sulphate and hydrogen gas are formed.
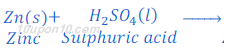
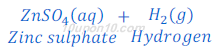
Hydrogen gas is utilized in hydrogenation of oil.
Hydrogen gas burns with pop sound. Thus when a burning candle is brought near the gas evolved, if it burns with pop sound, this confirms the presence of hydrogen gas.