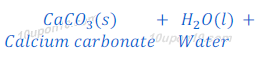Acid Base & Salt - Class 10th Science
Chemical Properties-3
Reaction of acid with hydrogen carbonates (hydrogen carbonates are also known as bicarbonates):
Examples:
(1) When sodium bicarbonate (sodium hydrogen carbonate) reacts with hydrochloric acid, sodium chloride is formed along with carbon dioxide and water.
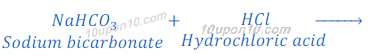

Sodium hydrogen carbonate is also known as baking soda, baking powder, bread soda or bicarbonate of soda.
(2) When sodium carbonate (sodium hydrogen carbonate) reacts with sulphuric acid, sodium sulphate is produced along with carbon dioxide and water.
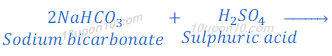
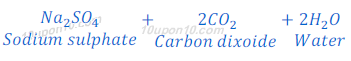
Characteristic test for carbon dioxide gas:
When carbon dioxide, produced in this or above reactions, is passed through lime water, lime water turns milky because of formation of insoluble calcium carbonate. Calcium carbonate formed in this reaction as white precipitate.
The chemical name of lime water is calcium hydroxide.
This is the characteristic test for carbon dioxide gas.
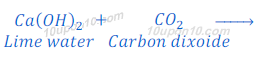
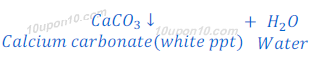
In this reaction; downwards arrow indicates the formation of precipitate.
When the excess of carbon dioxide gas is passed through the solution of this reaction mixture, the white (milky) colour of lime water vanishes out. This happens because of formation of calcium hydrogen carbonate (calcium bicarbonate) which is soluble in water.