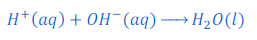Acid Base & Salt - Class 10th Science
Base in Water Solution
When a base is dissolved in water it dissociates hydroxide ions (OH—).
Example:
When sodium hydroxide is dissolved in water it produces hydroxide ion.
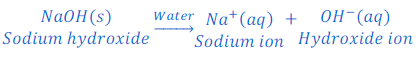
When calcium hydroxide is dissolved in water, it produces hydroxide ion.
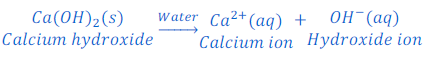
When potassium hydroxide is dissolved in water it produces hydroxide ion.
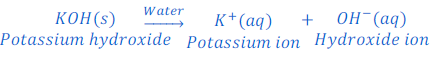
When ammonium hydroxide is dissolved in water, it produces hydroxide ion.
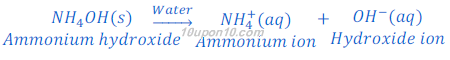
Magnesium hydroxide, a base, is not fully soluble in water, but it gives hydroxide ion when dissolved in water.
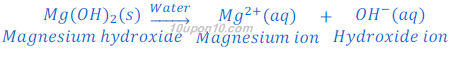
Ammonia gas is basic in character and soluble in water. When ammonia gas is dissolved in water it gives ammonium hydroxide. This ammonium hydroxide dissociates hydroxide ion.
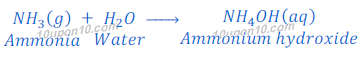
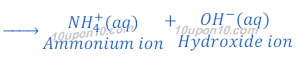

Is a base produce hydroxide ion in absence of water?
Similar to acid base also not produced hydroxide ion in absence of water. However base produce hydroxide ions in molten state. This is the cause that a base conducts electricity in molten form.
How neutralization reaction takes place:
Acid produces hydrogen ions and base produces hydroxide ions. Thus when an acid and base react, hydrogen ions produced by acid and hydroxide ions produced by base combined together and water molecules are formed. Because of formation of water molecule by hydrogen ion and hydroxide ion reaction mixture becomes neutralize. Along with water molecule respective salt is also formed in a neutralization reaction.
In other words hydrogen ions of an acid and hydroxide ions of a base neutralize each other.
Acid + Base ⇒ Salt + Water
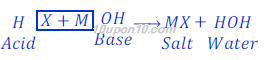
Where, X is anion (negative ion) of an acid and M is the cation (positive ion) of a base.