Carbon and Its Compounds - Class 10th Science
Chain, Branches and Rings
Carbon atoms forms compounds with different type arrangement, such as long chain, branched chain and cyclic chain structures.
Structural Isomers
Hydrocarbons having same molecular formula but different structural formula are called Structural Isomers.
Example:
Butane:
Molecular formula of hydrocarbon having four carbon atoms = C4H6
Name of this compound: Butane
Butane can have following two types of structural formula. This means four carbon atoms can be arranged in following two ways.
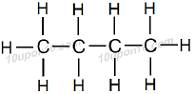
Name of this hydrocarbon is Butane or n–butane
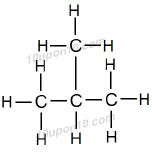
Name of this hydrocarbon is Isobutane. In this one carbon atom is arranged as branched chain.
These two hydrocarbons n–butane and isobutene has same molecular formula but different structural formula and are called structural isomers.
Pentane:
Molecular Formula of pentane = C5H12
In pentane carbon atoms can arrange into three ways, first forming straight chain and second forming one side chain and third forming two side chains.
Straight chain of pentane
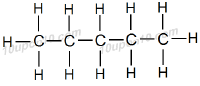
Name of this hydrocarbon is n–pentane.
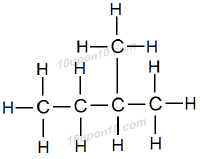
This hydrocarbon has also five carbon atoms. But one carbon atom is attached as branched chain.
Thus, name of this hydrocarbon is Iso–pentane or simply Isopentane.
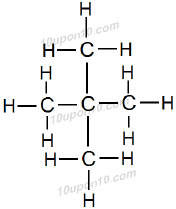
This hydrocarbon also has five carbon atoms but two carbon atoms are attached as branched chain. The name of this hydrocarbon is neo–pentane or simply neopentane.
Here, n–pentane, isopentane and neopentane are structural isomers having similar molecular formula C5H12
Cyclic Hydrocarbon (Hydrocarbon having ring structures)
When carbon atoms form chain in the form of ring, such hydrocarbon is called cyclic hydrocarbon.
Example:
Cyclopentane: Molecular formula of cyclopentane is C5H10
Structural formula of cyclopentane:
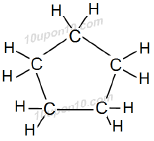
Cyclohexane: Molecular formula of cyclohexane is C6H12
Structural formula of cyclohexane:
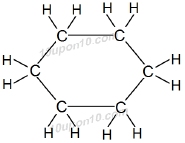
Benzene:
The molecular formula of benzene is C6H6
Structural formula of benzene:
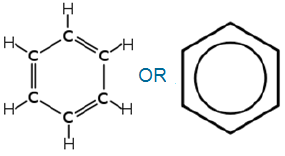
Structural formula of benzence can be written in above two ways.
Functional Group
Carbon forms compound with atoms other than hydrogen also. For example halogens, oxygen, nitrogen and sulphur.
In a carbon chain one or more hydrogen atom can be replaced by these atoms so that the valency of carbon remains satisfies. Such atoms are called heteroatoms.
Carbon also combines with group of atoms having specific propreties. Such groups of atoms containing specific properties regarless of the length of carbon chain are called FUNCTIONAL GROUPS.
Example: List of Functional Groups
Halo group: –Cl (Chloride), –Br (Bromide), –I (Iodide) and –F (Fluoride) are collectively called halo group or halogen group.
Alcohol: –OH (Alcohol group)
Aldehyde: –COH (Aldehyde group)
Ketone: –CO– (Ketone group)
Carboxylic Acid: –COOH (Carboxylic acid group)
These functional groups attached with carbon chain and form compounds which have chemical properties completely different from parent hydrocarbons.
Carbon chain containing same functional groups have similar chemical properties.
Homologous Series
Series of carbon compounds having same general formula regardless of functional groups are called HOMOLOGOUS SERIES. Members of compounds of a homologous series have similar chemical properties.
Example:
CH4 (Methane), C2H6(Ethane), C3H8(Propane), C4H10(Butane) and so on forms a homologus seris.
CH3OH, C2H5OH, C3H7OH, and so on forms a homologous series. Members of this homologous series are alcohols.
CH3COH, C2H5COH, C3H7COH, and so on forms a homologous series. Members of this homologous series are aldehydes.
CH3COOH, C2H5COOH, C3H7COOH, and so on forms a homologous series. Members of this homologous series are carboxylic acids or simply acids.
Properties of Homologous Series
Each members of a homologous series differs from its successive members by –CH2.
Each members of a homologous series differs by atomic mass of 14 u.
Example:
CH4 and C2H6 – these differes by – CH2 and difference of atomic mass between them is equal to 14
[Atomic mass of carbon (C) = 12 and Atomic mass of Hydrogen (H) = 1
∴ Atomic mass of CH4 = 12 + (1 x 4) = 16 and
and Atomic mass of C2H6 = (12 x 2) + (1 x 6) = 30
∴ Difference of atomic mass of Ethane and Methane = 30 – 16 = 14 u]
CH3OH and C2H5OH – these differes by – CH2 and difference of atomic mass between them is equal to 14
[Atomic mass of CH3OH = 12 + (1 x 3) + 16 + 1 = 31
And atomic mass of C2H5OH = (12 x 2) + (1 x 5) + 16 + 1 = 45
Thus, difference of atomic mass = 45 – 32 = 14 u]
C2H5COOH and C3H7COOH – these differes by – CH2 and difference of atomic mass between them is equal to 14
Allotropes of Carbon
The property of some elements to exist in different form in the same physical state is called Allotorhy and forms of elements are called Allotropes. Allotropes have different chemical properties but almost same chemical properties.
Carbon occurs in different forms having widely difference in their chemical properties. These forms carbon are called Allotropes of Carbon.
Diamond, Graphide, Charcoal, Fullerenes are some of the Allotropes of Carbon.
These allotropes of carbon exist because of bonding in carbon atoms in different fashion.
Diamond
In diamond each carbon atom is bonded to four other carbon atoms and forms a rigid three dimensional structure. Diamond is hardest natural occurring substance so far known.
Graphite
In graphite each carbon atom is bonded to three other carbon atoms in same plane, this forms a hexagonal structure. Graphite being a non-metal is good conductor of electricity and have shiny surface.
Fullerenes
Fullerenes are other types of allotropes of carbon. C-60 was the first fullerene was identified as one of the allotropes of carbon. Carbon atoms arrange in Fullerene is such a manner that it gives the shape of a football.
Because a US architect Buckminster Fuller designed the structure of c-60 similar to geodestic dome, thus molecule is known as fullerene.