Chemical Reactions and Equations - Class 10th Science
Balanced Chemical Equations
Law of Conservation of Mass: Law of conservation states that mass can neither be created nor be destroyed in a chemical reaction.
This means that total mass of elements present in reactants must be equal to the total mass of elements present in product in a chemical reaction.
All chemical reaction must have to obey the Law of Conservation of Mass. Thus, in order to satisfy the Law of Conservation of Mass, a chemical reaction is needed to be balanced.
Balancing a Chemical Equation
Let, the reaction of burning of magnesium ribbon in air.
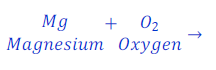
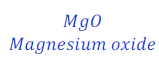
To balance the given chemical equation, given steps are followed:
Step: 1: Write the number of atoms of each of the elements in a table.
| Number of atoms in each side | ||
|---|---|---|
| Elements | No. of atoms in reactants (LHS) | No. of atoms in product (RHS) |
| Mg | 1 | 1 |
| O | 2 | 1 |
Here, number of Mg (Magnesium) in reactant and product is equal i.e. equal to one (1).
Number of O2 (Oxygen) in reactant is equal to two(2) and in product is equal to one.
This means, number of oxygen is not balanced in both sides of the chemical equation.
Step: II:Add 2(two) before the product, MgO(Magnesium oxide) in order to balance the chemical equation.
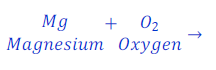
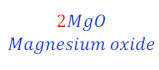
Step: III:Now, again draw the table and observe the number of atoms of each of the elements in reactant and product.
| Number of atoms in each side | ||
|---|---|---|
| Elements | No. of atoms in reactants (LHS) | No. of atoms in product (RHS) |
| Mg | 1 | 2 |
| O | 2 | 2 |
Now, number of magnesium in reactant is equal to 1(one) and in product became equal to 2(two).
And, number of oxygen in reactant and product became equal, i.e. equal to two (2).
Step: IV:Add two before, Mg(Magnesium) in reactant to balance it in both sides.
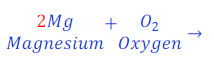
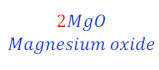
Step: V:Again draw the table and observe the number of atoms in both sides, i.e. in reactant and product sides.
| Number of atoms in each side | ||
|---|---|---|
| Elements | No. of atoms in reactants (LHS) | No. of atoms in product (RHS) |
| Mg | 2 | 2 |
| O | 2 | 2 |
Now, number of magnesium in reactant and product became equal to 2 (two).
And number of oxygen (O2) in reactant and product also became equal to two (2).
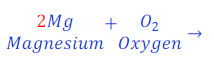
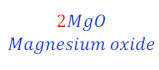
Since, number of atoms of each of the elements in both sides became equal, hence, given chemical equation is balanced now.
Example : 2 :
Zinc + Sulphuric acid → Zinc sulphate + Hydrogen
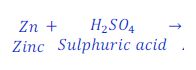
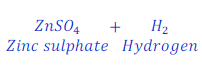
Step: 1:Draw a table to compare the atoms of each of the elements present in reactant and product.
| Number of atoms in each side | ||
|---|---|---|
| Elements | No. of atoms in reactants (LHS) | No. of atoms in product (RHS) |
| Zn | 1 | 1 |
| H | 2 | 2 |
| S | 1 | 1 |
| O | 4 | 4 |
Here, it is clear that
Number of atoms of each of the element in reactant and product is equal i.e. no need to balance them. This means this is a balance chemical equation.
Example: 3:
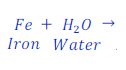
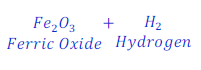
Step: 1:Draw a table with atoms of each elements present in reactants and products to compare them.
| Number of atoms in each side | ||
|---|---|---|
| Elements | No. of atoms in reactants (LHS) | No. of atoms in product (RHS) |
| Fe | 1 | 2 |
| H | 2 | 2 |
| O | 1 | 3 |
Step: 2:In order to balance a chemical equation, start with the atom of element having maximum number.
Here, number of oxygen is equal to three(3) in product and is equal to 1 (one) in reactant.
So, Multiply, H2O(water) in reactant with 3 (three) to balance number of oxygen atom both side.
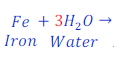
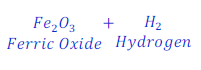
| Number of atoms in each side | ||
|---|---|---|
| Elements | No. of atoms in reactants (LHS) | No. of atoms in product (RHS) |
| Fe | 1 | 2 |
| H | 3 x 2 = 6 | 2 |
| O | 1 x 3 = 3 | 3 |
Step: 3:Now, to make the number of hydrogen atoms equal both side, multiply, H2 (hydrogen) in product by 3(three)
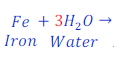
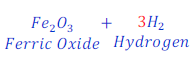
Step: 4:Now multiply Fe(Iron) present in reactant by 2(two) to make it equal to present in product.
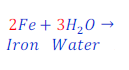
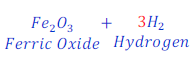
| Number of atoms in each side | ||
|---|---|---|
| Elements | No. of atoms in reactants (LHS) | No. of atoms in product (RHS) |
| Fe | 2 | 2 |
| H | 3 x 2 = 6 | 2 x 3 = 6 |
| O | 1 x 3 = 3 | 3 |
Now, it is clear that all atoms of each of the elements became equal in both sides. Hence, this equation became balanced now.