Chemical Reactions and Equations - Class 10th Science
Displacement Reaction
When a more reactive element replaces or displaces a less reactive element from its compound while reacting it is called displacement reaction.
In a displacement reaction both metals and non-metals take part. Displacement reaction is also known as Single Displacement Reaction because in this only one displacement takes place.
General Reaction for displacement reaction
A displacement or single displacement reaction can be represented in following way:
Example:1:
When iron nail is kept dipped in the solution of copper sulphate for some time, iron displaces copper from copper sulphate and forms ferrous sulphate.
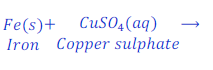
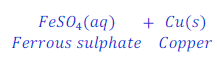
In this reaction, iron is more reactive than copper and hence displaces copper from copper sulphate and makes ferrous sulphate (iron sulphate).
Example: 2:
When Zinc is kept dipped in the solution of copper sulphate for some time, zinc replaces copper from copper sulphate and makes zinc sulphate.
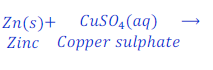
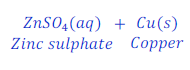
In this reaction zinc is more reactive than copper, and hence replaces copper from copper sulphate and makes zinc sulphate.
Example: 3:
When Lead is kept dipped in the solution of copper chloride for some time, lead chloride is formed after displacement of copper from the solution of copper chloride.
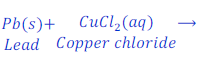
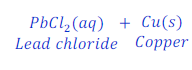
In this reaction, lead is more reactive than copper and hence replaces copper from the copper chloride and makes lead chloride.
Example: 4:
When copper is kept dipped in the solution of silver nitrate, copper replaces silver from the solution of silver nitrate and makes copper nitrate.
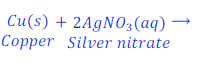
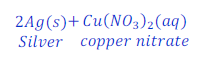
In this reaction, copper is more reactive than silver and hence displaces silver from silver nitrate.
Example: 5:
When calcium reacts with water, it replaces hydrogen from water and forms calcium hydroxide.
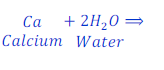
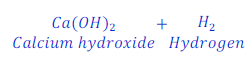
In this reaction, calcium is more reactive than hydrogen and hence replaces hydrogen from water.
Example: 6:
When sodium reacts with aluminium chloride, it replaces aluminium from the solution of aluminium chloride and forms sodium chloride.

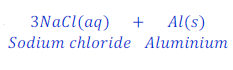
In this sodium is more reactive than aluminium and hence, replaces aluminium from the solution of aluminium chloride.
Example: 7:
When Aluminium is kept dipped in the solution of sodium chloride, no reaction takes place.
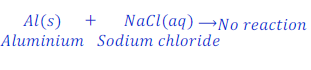
In this case aluminium is very less reactive than sodium and hence could not replaces sodium from the solution of sodium chloride.
Example: 8:
When copper is kept dipped in the solution of zinc sulphate, no reaction takes place.
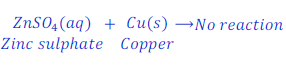
This happens because copper is less reactive than zinc.
Use of Displacement Reaction:
Displacement reactions are used in electroplating of metals, such as