Acid Base & Salt - Class 10th Science
What is Salt
Most common salt is common salt, i.e. sodium chloride (NaCl). Salt is produced in laboratory by the neutralization reaction of acid and base. Potassium sulphate, sodium sulphate, sodium nitrate, sodium carbonate, etc. are examples of some salts other than sodium chloride.
A salt contains two types of ions, i.e. positive and negative. For example in NaCl, Na+ is positive ion and Cl — is negative ion.
Generally, a salt is neutral in nature as a salt is formed by the combination of cation and anion.
Example:
When sodium hydroxide (a base) reacts with hydrochloric acid, water and sodium chloride (a salt) are formed.
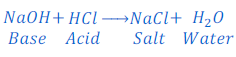
In this reaction sodium ion from sodium hydroxide and chloride ion from hydrochloric acid form sodium chloride by combining together. Hydroxide ion (OH—) from Sodium hydroxide, and hydrogen ion (H+) from hydrochloric acid are replaced and combine together to form water.
In similar manner lot of salts are formed.
Naming of salt:
Salts are named after the acid and base because of which they formed.
Cation, which is first part of salt is named after base and anion, which is second part of salt is named after acid.
Examples:
(1) Sodium chloride (a salt) is formed after the neutralization reaction of sodium hydroxide (a base) and hydrochloric acid (an acid). It got its name as sodium (first part) after sodium hydroxide and chloride (second part) after hydrochloric acid (hydrogen chloride).
(2) Calcium nitrate is formed after the reaction of calcium hydroxide and nitric acid. Therefore, in Calcium nitrate (a salt), calcium comes from calcium hydroxide and nitrate comes from the nitric acid.
(3) Potassium sulphate is formed after the reaction between potassium hydroxide and sulphuric acid (hydrogen sulphate). In this salt potassium comes after potassium hydroxide and sulphate comes after the sulphuric acid.
Thus,
(a) Because of hydrochloric acid, chloride salts are formed.
Example:
sodium chloride (NaCl), calcium chloride (CaCl2), potassium chloride (KCl), Magnesium chloride (MgCl2), Copper chloride (CuCl2), Zinc chloride (ZnCl2), Aluminium chloride (AlCl3), Barium chloride (BaCl2), etc.
(b) Salts formed by sulphuric acid (H2SO4) are called sulphate salts.
Example:
Sodium sulphate (Na2SO4), potassium sulphate (K2SO4), Calcium sulpahte(CaSO4), Magnesium sulphate(MgSO4), copper sulphate(CuSO4), zinc sulphate(ZnSO4), Iron sulphate(FeSO4), Aluminium slupahte(Al2(SO4)3), Ammonium sulpahte ((NH4)2SO4), etc.
(c) Salts formed by nitric acid are called nitrate.
Example:
Sodium nitrate(NaNO3), Calcium nitrate (CaNO3), Potassium nitrate(KNO3), Ammonium nittrate (NH4NO3), Beryllium nitrate (BeNO3), Magnesium nitrate (Mg(NO3)2), Zinc nitrate (Zn(NO3)2), etc.
(d) Salts formed because of carbonic acid are called carbonates.
Example:
Sodium carbonate (Na2CO3), Calcium carbonate (CaCO3), Magnesium carbonate (MgCO3), Potassium carbonate (K2CO3), Beryllium carbonate (BeCO3), Copper carbonate(CuCO3), etc.
(e) Similarly, salts formed by acetic acid are called acetates.
Common Characteristics of salts:
Most of the salts are solid.
Salts can be transparent or opaque.
Salts are generally soluble in water.
Most of the salts dissociate ions in aqueous solution, thus they conduct electricity in their aqueous solution. Salts in their molten state also conduct electricity.
A salt has two parts, one is cation (positively charged) and other is anion (negatively charged).
Since, salts are formed after the neutralization reaction, thus, a salt is electrically neutral in general.
Most of the salts are coulerless, but many of them are coloured.
Examples of some coloured salts:
Yellow (sodium chromate)
Orange (potassium dichromate)
Red (potassium ferricyanide)
Mauve (cobalt chloride hexahydrate)
Blue (copper sulfate pentahydrate, ferric hexacyanoferrate)
Purple (potassium permanganate)
Green (nickel chloride hexahydrate)
Colorless (magnesium sulfate heptahydrate) may appear white when powdered or in small pieces