Acid Base & Salt - Class 10th Science
NCERT Exemplar Solution:Short Answer1
Question: 31. Match the acids given in Column (A) with their correct source given
| Column(A) | Column(B) |
|---|---|
| (a) Lactic acid | (i) Toamto |
| (b) Acetic acid | (ii)Lemon |
| (c) Citric acid | (iii) Vinegar |
| (d) Oxalic acid | (iv) Curd |
Answer:
(a) – (iv)
(b) – (iii)
(c) – (ii)
(d) – (i)
Question: 32. Match the important chemicals given in Column (A) with the chemical formulae given in Column (B)
| Column(A) | Column(B) |
|---|---|
| (a) Plaster of Paris | (i) Ca(OH)2 |
| (b) Gypsum | (ii)CaSO4.1/2 H2O |
| (c) Bleaching Powser | (iii) CaSO4.2H2 |
| (d) Slaked Lime | (iv) CaOCl2 |
Answer:
(a) – (ii)
(b) – (iii)
(c) – (iv)
(d) – (i)
Question: 33. What will be the action of the following substances on litmus paper?
Dry HCl gas, Moistened NH3 gas, Lemon juice, Carbonated soft drink, Curd, Soap solution.
Answer:
Dry HCl: No action
Moistened NH3 gas: Turn blue litmus to red
Lemon Juice: Turn blue litmus to red
Carbonated soft drink: Turn blue litmus to red
Curd: Turn blue litmus to red
Soap solution: Turn red litmus to blue.
Question: 34. Name the acid present in ant sting and give its chemical formula. Also give the common method to get relief from the discomfort caused by the ant sting.
Answer:
Formic acid (methanoic acid) present in ant sting
Chemical Formula of formic acid: HCOOH
Common method to get relief from the discomfort caused by ant sting: Baking soda is rub over the place of ant stung which neutralizes the acid present in the sting and gives relief from discomfort.
Question: 35. What happens when nitric acid is added to egg shell?
Answer:
Egg shell contains mainly calcium carbonate.
Thus when nitric acid is added to egg shell, it forms carbon dioxide and calcium nitrate.

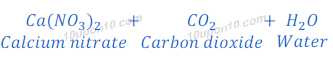
Question: 36. A student prepared solutions of (i) an acid and (ii) a base in two separate beakers. She forgot to label the solutions and litmus paper is not available in the laboratory. Since both the solutions are colourless, how will she distinguish between the two?
Answer:
It is assumed that other indicators will be available in laboratory. Thus, student will use other indicators, such as phenolphthalein.
Phenolphthalein turns pink with basic solution and remains colorless with acidic solution.
Thus, by using phenolphthalein, student can label the beakers (i) and (ii) having solution with acid and base.
Question: 37. How would you distinguish between baking powder and washing soda by heating?
Answer:
Baking powder (sodium hydrogen carbonate) produces carbon dioxide gas at very low temperature, while washing soda (sodium carbonate) does not produce carbon dioxide at low temperature, but washing soda produces carbon dioxide gas at very high temperature.
Thus, to distinguish between baking powder and washing soda, they are heated in separate test tubes.
Gases produced by them are passed through lime water.
Gas coming out from test tube which turns lime water milky is baking powder and which does not is washing soda.