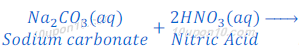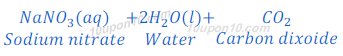Acid Base & Salt - Class 10th Science
Chemical Properties-2
How do metal carbonates and Metal Hydrogen carbonates react with Acids?
When acid reacts with metal carbonate or metal hydrogen carbonate, corresponding salt, carbon dioxide gas and water are formed.
Metal Carbonate + Acid → Salt + Carbon dioxide + Water
Metal Hydrogen Carbonate + Acid → Salt + Carbon dioxide + Water
Example:
Reaction of acids with metal carbonate:
When sodium carbonate reacts with hydrochloric acid, sodium chloride is formed along with carbon dioxide and water.
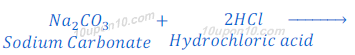
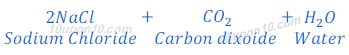
When sodium carbonate reacts with sulphuric acid, sodium sulphate, carbona dioxide and water are formed.
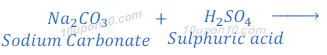
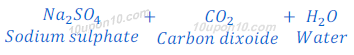
When magnesium carbonate reacts with hydrochloric acid, magnesium chloride, carbon dioxide and water are formed.


When calcium carbonate reacts with hydrochloric acid, calcium chloride, carbon dioxide and water are formed.
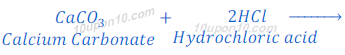
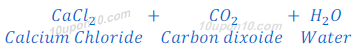
When calcium carbonate reacts with sulphuric acid, calcium sulphate, carbon dioxide and water are formed.
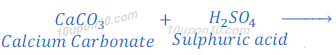
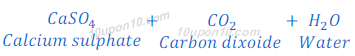
When sodium carbonate reacts with nitric acid, sodium nitrate, water and carbon dioxide are formed.