Chemical Reactions and Equations - Class 10th Science
Short Answers: 2
Question: 16. What is rancidity?
Answer: Spoilage of food because of oxidation of fats present in them is called rancidity.
Question: 17. What is corrosion of metals?
Answer: Degradation of metals because of the formation of a layer of respective oxides, sulphide, etc. over them is called corrosion of metals.
Question: 18. Why silver metals appear black if kept in open for some days?
Answer: Silver metals appear black because of formation of a layer of sulphide over them if kept open for some days.
Question: 19. Why it is advisable to show the physical states of substance when writing a chemical equation.
Answer: Writing the physical states of substances makes chemical equations more informative.
Question: 20. Why displacement reaction is also known as single displacement reaction?
Answer: In displacement reaction only one atom or ion is displaced, that’s why it is also known as single displacement reaction.
Question: 21. Which substance is used in white washing?
Answer: Calcium hydroxide (slaked lime) is used in white washing.
Question: 22. What is endothermic reaction?
Answer: Chemical reactions, in which heat is absorbed, are called endothermic reactions, for example, decomposition of calcium carbonate.
Question: 23. What is exothermic reaction?
Answer: Chemical reactions, in which heat is evolved, are called endothermic reactions, for example, reaction of calcium oxide with water.
Question: 24. When quick lime reacts with water, it gives calcium hydroxide. Write the chemical equation of this reaction.
Answer:
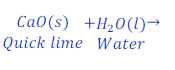
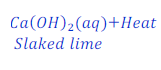
Question: 25. When calcium hydroxide reacts with carbon dioxide gas, it gives calcium carbonate. Write the chemical equation for this.
Answer:
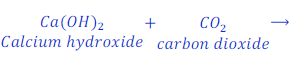
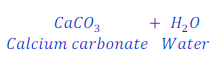
Question: 26. Coal gives carbon dioxide and heat when burns in the presence of air. Write the chemical equation for this reaction.
Answer:
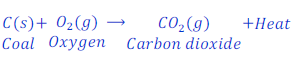
Question: 26. Water is formed when hydrogen gas reacts with oxygen gas. Write the chemical equation for this reaction.
Answer:
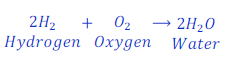
Question: 27. Magnesium oxide is formed when magnesium ribbon is burns in the presence of oxygen. What will be the chemical equation for this reaction?
Answer:
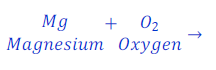
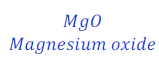
Question: 28. What happens when water is put under electrolysis? Write the chemical equation for this.
Answer:

Question: 29. When lime stone is heated it decomposes into quick lime and carbon dioxide gas. Write the chemical equation for this reaction.
Answer:
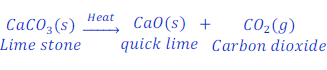
Question: 30. Write the thermal decomposition reaction of ferrous sulphate.
Answer: