Chemical Reactions and Equations - Class 10th Science
Decomposition Reaction
When a single compound breaks down into two or more substances, it is called a decomposition reaction. Decomposition means to break down.
Decomposition reaction is just opposite of combination reaction.
Example:1:
When electric current is passed through water, it goes under decomposition reaction and dissociates hydrogen and oxygen.

Example:2:
When limestone (calcium carbonate) is heated, it goes under decomposition reaction and gives quick lime(calcium oxide) and carbon dioxide gas.
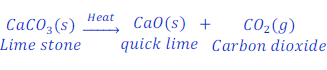
Decomposition of calcium carbonate (quick lime) is used in various industries, like cement.
Example:3:
When Ferrous sulphate is heated, it goes under decomposition reaction and produces ferric oxide, sulphur dioxide and sulphur trioxide gas.

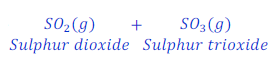
The crystal of ferrous sulphate has green colour. When crystal of ferrous sulphate is heated, it loses water and its green colour vanishes out because of formation of ferric oxide.
Example:4:
When lead nitrate is heated, it gives lead oxide, nitrogen oxide gas and oxygen gas after decomposition.
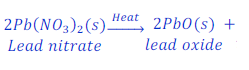
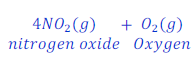
Example: 5 :
When silver chloride is placed or exposed in sunlight, it absorbs heat from sunlight and decomposes into silver and chlorine gas.

Example: 6:
When silver bromide is placed or exposed in sunlight, it absorbs heat from sunlight and decomposes into silver and bromine gas.

Decomposition of silver chloride and silver bromide is used in black and white photography. Photograph paper contains a coat of either silver chloride or silver bromide and when exposed to sunlight turns black because of formation of silver and leaves the impression of photograph.