Metals & Non-metals - Class 10th Science
Corrosion
Gradual eating up of metals by the formation of coating of unwanted compound because of reaction of oxygen, carbon dioxide, sulphur, acid, etc. present in atmosphere, over them is called corrosion.
Example:
When iron metal comes in contact with oxygen present in moist air, a brown coat deposited over it because of formation of iron oxide. This is called rusting of iron.
When silver metal comes in contact with sulphur present in air, a black coat is deposited over it because of formation of silver sulphide. This is called corrosion or tarnishing of silver.
When copper comes in contact with carbon dioxide present in air, a green coat is deposited over it because of formation of copper carbonate. This is called corrosion or tarnishing of copper.
Rusting of iron
When articles made of iron come in contact with moist air, oxygen present in moist air reacts with iron and form layer of iron oxide over them. Iron oxide is porous in nature which falls after some time and next layer of iron start reacting with oxygen present in moist air. Gradually whole of article made of iron metal is converted into metal oxide. Because of this process, i.e. conversion of iron into iron oxide after reaction of oxygen present in moist air, whole of the article made of iron is eaten up or gets destroyed. Iron oxide so formed is known as rust and this process is called RUSTING OF IRON.
Reaction involved in Rusting of Iron
When iron reacts with water and oxygen it forms iron (III) hydroxide or ferric hydroxide. The ferric hydroxide after dehydration turns into ferric oxide (rust).
Iron + Water + Oxygen → Rust
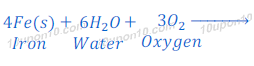

Ferric hydroxide gets dehydrated after some times and produces rust. Chemical formula of rust is Fe2O3n . H2O.
Chemical name of RUST is hydrated iron (III) oxide or Ferric oxide.


Conditions for Rusting of Iron
For rusting of iron, iron should be come in contact with oxygen and water. In the absence of any one, i.e. water or oxygen, iron does not get rusted.
Thus, when iron metal comes in contact with moist air, it gets rusted.
Thus, if there were no moisture present in air, iron would not be rusted. Hence, by preventing either oxygen or water to come in contact with iron rusting can be prevented.
To observe the rusting of iron
Rusting of iron can be observed by a simple activity.
- Take three clean and dry test tubes and labeled them as A, B and C.
- In test tube "A" put some anhydrous calcium chloride and then put some iron nails and close its mouth using a cork.
- In test tube "B" take some boiled water and put iron nails and then pour a layer of oil and close with a cork.
- In test tube "C" take some tap water and put iron nails and close with a cork.
- Leave all the three test tubes for about 10 to 15 days.
After about 15 days observe nails present in test tubes.
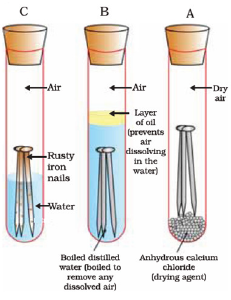
Ref: Image taken from NCERT Book
You will observe that no rust has been deposited over nails kept in test tube 'A' and 'C'. While nails kept in test tube "C" got rusted.
This happens because:
(a) In test tube "A" anhydrous calcium chloride absorbs all the moisture present in the vacant space in it. Because of absence of moisture, i.e. any water, nails kept in it do not react with water and hence did not get rusted.
(b) In test tube "B" because of boiling, air present in water is expelled out. Layer of oil prevents pushing of any oxygen in water. And thus no oxygen present in water to react with iron nails present in it. That's why iron nails in test tube 'B' did not rust.
(c) In test tube "C" tap water contains oxygen dissolved in it. This oxygen and water react with iron nails and rust deposited over them.
This activity shows that both water and oxygen is necessary for rusting of iron.
Prevention of rusting of iron
Since, oxygen and water both are necessary for the formation of ferric oxide, i.e. rust. Thus, by preventing either oxygen or water from coming in contact with iron, rusting of iron can be prevented.
There are many methods to prevent rusting of iron.
Painting
By painting of articles made of iron, rust can be prevented.
Making of coat of paint over the articles made of iron prevent iron from come in contact with moisture present in air and this prevent rusting.
This is the cause that grills, car’s body, steel furniture, bridges made of iron, etc. and many other articles made of iron are painted at regular basis.
Applying of grease or oil
Applying of a coat of grease or oil also prevent the articles made of iron to come in contact with moisture present in air. And by applying of a coat of grease or oil rusting of iron is prevented.
This is the cause that coat grease is applied over the chain of cycle and other many machine parts which prevent them from getting rusted.
Galvanisation
Galvanisation is the process of depositing a layer of zinc over articles made of iron. A thin layer of zinc over iron metal, prevent it to come in contact with moist air, and rusting is prevented.
However, zinc is more reactive than iron, but when zinc comes in contact with oxygen present in air, a thin layer of zinc oxide is formed over it which further prevents the zinc to react with oxygen present in air.
This is the cause that pipes used for water supply, buckets made of iron, etc. are usually galvanized to prevent rusting.
Tin or chromium plating
Depositing of a layer of tin or chromium over articles made of iron or other metal is called tin or chromium plating.
Thin layer of tin or chromium metal is deposited over iron by the process of electroplating.
This layer of tin or chromium which are resistant to corrosion prevents articles made of iron to come in contact with moisture present in air, which prevents rusting.
This is the cause that many articles made of iron come with chromium or tin plating, such as cycle rim, tiffin boxes, water taps, handle of bicycles, car bumpers, etc.
Apart from preventing of rust chromium or tin plating gives articles good and shiny look.
Alloying
Alloying is a process of making homogeneous mixture of two or more metals.
Stainless steel is made by mixing of iron, chromium and nickel. Alloying prevents rusting by making iron corrosion resistant.
This is the cause that cooking utensils are made of stainless steel, which is alloy of iron.
Corrosion or tarnishing of Aluminium
Aluminium is more reactive than iron. Thus when aluminium comes in contact with moist air present in atmosphere, aluminium oxides is formed and gets deposited in the form of a thin layer over it. This thin layer of aluminium oxide makes the appearance of aluminium metal dull. But aluminium oxide does not react with oxygen, thus this thin layer of aluminium oxide prevents further corrosion of articles made of aluminium.
Depositing of thin layer of aluminium oxide over aluminium metal after reaction with oxygen present in air is called corrosion of aluminium or tarnishing of aluminium.
Corrosion of copper
When copper metal is exposed to air for a long time, a green coat is deposited over it. This green coat makes the appearance of copper dull.
This happens because of reaction of copper with carbon dioxide and water present in air. Because of this reaction a thin layer of copper carbonate is deposited over copper metal.
Usually we see copper coins and other articles made of copper become greenish or blackish after some times because of exposure with atmosphere.
This is called corrosion of copper or tarnishing of copper.
Corrosion of silver
When silver metal comes in contact with sulphur present in air, it becomes dull. This happens because of formation of layer of silver sulphide over silver metal. Formation of layer of silver sulphide makes silver metal blakish.
This is called corrosion of silver or tarnishing of silver.
Ornaments, coins, etc. made of silver often tarnished when left for long time.
Alloy
Alloy is the homogeneous mixture of two or more than two metals or non-metals. Alloying improves the qualities of metal, such as strength, resistance to corrosion, ductility, malleability, etc.
Examples of alloy: stainless steel is the alloy of iron, chromium and nickel. Mixing of these metals with iron increase the strength of iron and make it corrosion resistant.
Carbon is mixed with iron to make the iron hard and stronger.
Bronze
Bronze is the alloy of copper and tin.
Bronze is made by mixing 88% of copper and 12% of tin. By mixing of tin copper metal is prevented from getting corroded. Bronze has been used for ancient time.
Bronze is used in making of ship propellers, submerged bearings, statues, etc.
Brass
Brass is the alloy of copper and zinc. By mixing zinc, malleability of copper is increased, so brass is more malleable than copper. Brass is corrosion resistance because of mixing of zinc.
Brass is used in making statues, musical instruments, etc.
Alloying of gold
Gold is very soft metal because of which making of jewelry is difficult with pure gold. Thus, to increase its hardness, copper or silver is mixed with gold.
Pure gold is said to be of 24 carats. Gold jewelries available in market are usually of 22 carat. This means 22 carat of gold contains 2% of copper or silver and 98% of gold.
Amalgam
In alloy if one of the metals is mercury, then alloy is called amalgam. Thus, amalgam is the homogeneous mixture of mercury and other metals.
Electrical conductivity and melting point of Alloy
Alloy has less electrical conductivity and lower melting point than that of pure metal.
Example:
Copper is good conductor of electricity but brass, which is an alloy of copper, zinc is not a good conductor of electricity.
Bronze, which is an alloy of copper and tin is not a good conductor of electricity.
Solder, which is an alloy of lead and tin has very low melting point. This is the cause that solder is used for welding electrical wires together.