Metals & Non-metals - Class 10th Science
Exemplar solution MCQ
Question: 1. Which of the following property is generally not shown by metals?
(a) Electrical conduction
(b) Sonorous in nature
(c) Dullness
(d) Ductility
Answer: (c) Dullness
Explanation: Metals have shiny appearance and not dull. Thus property of dullness is not shown by metals.
Question: 2. The ability of metals to be drawn into thin wire is known as
(a) ductility
(b) malleability
(c) sonorousity
(d) conductivity
Answer: (a) Ductility
Explanation: Dulctility is one of the properties of metals which enable metals to be drawn into thin wires.
Question: 3. Aluminium is used for making cooking utensils. Which of the following properties of aluminium are responsible for the same?
(i) Good thermal conductivity
(ii) Good electrical conductivity
(iii) Ductility
(iv) High melting point
(a) (i) and (ii)
(b) (i) and (iii)
(c) (ii) and (iii)
(d) (i) and (iv)
Answer: (d) (i) and (iv)
Explanation:
Aluminium has good thermal conductivity and high melting point. Good thermal conductivity enables aluminium to transfer heat to the cooking materials quickly and easily. High melting point enables aluminium to cook at high temperature.
Question: 4. Which one of the following metals do not react with cold as well as hot water?
(a) Na
(b) Ca
(c) Mg
(d) Fe
Answer: (d) Fe
Explanation: Na (sodium) and Ca(calcium) react with water vigorously while magnesium reacts with water less vigorously than that of calcium and sodium.
Iron (Fe) on the other hand does not react with cold as well as hot water instantly. However when iron is kept with water for long time, rusting of iron starts.
Question: 5. Which of the following oxide(s) of iron would be obtained on prolonged reaction of iron with steam?
(a) FeO
(b) Fe2O3
(c) Fe3O4
(d) Fe2O3 and Fe3O4
Answer: (c) Fe3O4
Explanation:
When steam is passed over iron for long time, it gives Iron oxide (Fe3O4) and hydrogen gas.
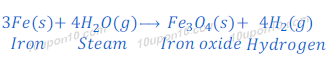
Question: 6. What happens when calcium is treated with water?
(i) It does not react with water
(ii) It reacts violently with water
(iii) It reacts less violently with water
(iv) Bubbles of hydrogen gas formed stick to the surface of calcium
(a) (i) and (iv)
(b) (ii) and (iii)
(c) (i) and (ii)
(d) (iii) and (iv)
Answer: (d) (ii) and (iv)
Explanation:
Calcium metal reacts with water vigorously and produces calcium hydroxide and hydrogen gas.
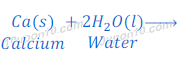
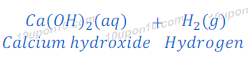
In this reaction of calcium and water, bubbles of hydrogen gas are formed and get stuck to the surface of calcium metal because of which calcium starts floating.
Reaction of calcium with water is exothermic but heat produced in this reaction is not sufficient so that hydrogen can catch fire.
Question: 7. Generally metals react with acids to give salt and hydrogen gas. Which of the following acids does not give hydrogen gas on reacting with metals (except Mn and Mg)?
(a) H2SO4
(b) HCl
(c) HNO3
(d) All of these
Answer: (c) HNO3
Explanation:
Metals react with nitric acid with another way. Nitric acid which is a strong oxidizing agent, oxidizes hydrogen produced in reaction to water and nitric acid itself is reduced to any of the nitrogen oxide [N2O (Nitrous oxide), NO (Nitric oxide) or NO2 (Nitrogen dioxide)].
Whereas magnesium and manganese reacts with dilute nitric acid and gives respective salt and hydrogen gas.
Question: 8. The composition of aqua-regia is
(a) Dil.HCl : Conc. HNO3 = 3 : 1
(b) Conc.HCl : Dil. HNO3 = 3 : 1
(c) Conc.HCl : Conc. HNO3 = 3 : 1
(d) Dil.HCl : Dil. HNO3 = 3 : 1
Answer: (c) Conc.HCl : Conc. HNO3 = 3 : 1
Explanation:
Aqua-regia is the mixture of concentrated hydrochloric acid and concentrated nitric acid in the ration of 3:1. Aqua-regia is very strong acid. Aqua-regia dissolves gold in it.
Question: 9. Which of the following are not ionic compounds?
(i) KCl
(ii) HCl
(iii) CCl4
(iv) NaCl
(a) (i) and (ii)
(b) (ii) and (iii)
(c) (iii) and (iv)
(d) (i) and (iii)
Answer: (b) (ii) and (iii)
Explanation:
Hydrochloric acid (HCl) and carbon tetrachloride (CCl4) form covalent compounds whereas sodium chloride (NaCl) and potassium chloride (KCl) form ionic compounds.
Question: 10. Which one of the following properties is not generally exhibited by ionic compounds?
(a) Solubility in water
(b) Electrical conductivity in solid state
(c) High melting and boiling points
(d) Electrical conductivity in molten state
Answer: (b) Electrical conductivity in solid state
Explanation:
Ions which can move are responsible for conduction of electricity. In solid state ions present in ionic compounds are not free to move, thus ionic compounds do not show electrical conductivity in solid state whereas ionic compounds show electrical conductivity in molten states.
Question: 11. Which of the following metals exist in their native state in nature?
(i) Cu
(ii) Au
(iii) Zn
(iv) Ag
(a) (i) and (ii)
(b) (ii) and (iii)
(c) (ii) and (iv)
(d) (iii) and (iv)
Answer: (c) (ii) and (iv)
Explanation:
Silver and gold fall at the bottom of reactivity series, this means gold and silver are not reactive or very less reactive. These metals are called noble metals. Since gold and silver are least reactive metals thus, these are found in free states in nature.
Question: 12. Metals are refined by using different methods. Which of the following metals are refined by electrolytic refining?
(i) Au
(ii) Cu
(iii) Na
(iv) K
(a) (i) and (ii)
(b) (i) and (iii)
(c) (ii) and (iii)
(d) (iii) and (iv)
Answer: (d) (i) and (ii)
Explanation:
Sodium and potassium are extracted by electrolytic reduction. Metals obtained after electrolytic reduction are in pure form.
Copper and gold obtained after extraction is in impure form. Copper and gold are refined by electrolytic refining methods.
Thus, option (d) (i) and (ii) is the correct option.