Metals & Non-metals - Class 10th Science
Exemplar Solution MCQs-3
Question: 25. An element A is soft and can be cut with a knife. This is very reactive to air and cannot be kept open in air. It reacts vigorously with water. Identify the element from the following
(a) Mg
(b) Na
(c) P
(d) Ca
Answer: (b) Na
Explanation:
Sodium is an element which so soft that can be cut using a knife. Sodium is so reactive that it reacts with oxygen or moisture present in air readily and reacts with water vigorously. Because of this sodium is stored in kerosene oil to prevent any reaction or accident due to reaction.
Thus option (b) Na (sodium) is the correct answer.
Question: 26. Alloys are homogeneous mixtures of a metal with a metal or nonmetal. Which among the following alloys contain non-metal as one of its constituents?
(a) Brass
(b) Bronze
(c) Amalgam
(d) Steel
Answer: (d) Steel
Explanation: Steel is an alloy of basically iron and carbon. Mixing of carbon gives strength to iron.
Question: 27. Which among the following statements is incorrect for magnesium metal?
(a) It burns in oxygen with a dazzling white flame
(b) It reacts with cold water to form magnesium oxide and evolves hydrogen gas
(c) It reacts with hot water to form magnesium hydroxide and evolves hydrogen gas
(d) It reacts with steam to form magnesium hydroxide and evolves hydrogen gas
Answer: (b) It reacts with cold water to form magnesium oxide and evolves hydrogen gas
Explanation: Reaction of metals and water produce hydrogen gas and respective hydroxide. For example sodium metal gives sodium hydroxide and liberates hydrogen gas when react with water.
Magnesium when reacts with water gives magnesium hydroxide and hydrogen gas and not magnesium oxide.
Thus, option (b) (b) It reacts with cold water to form magnesium oxide and evolves hydrogen gas is the correct answer.
Question: 28. Which among the following alloys contain mercury as one of its constituents?
(a) Stainless steel
(b) Alnico
(c) Solder
(d) Zinc amalgam
Answer: (d) Zinc amalgam
Explanation: In alloy is one of the constituent is mercury, the alloy is called amalgam.
Question: 29. Reaction between X and Y, forms compound Z. X loses electron and Y gains electron. Which of the following properties is not shown by Z?
(a) Has high melting point
(b) Has low melting point
(c) Conducts electricity in molten state
(d) Occurs as solid
Answer: (b) Has low melting point
Explanation: Ionic compounds are formed by the transfer of electrons. Ionic compounds conduct electricity in their molten state, occur as solid and have high melting and boiling points.
Low melting point is not the character of ionic compounds.
Given, compound Z is formed because of transfer of electrons, this means Z is an ionic compound which could not have low melting point.
Question: 30. The electronic configurations of three elements X, Y and Z are X – 2, 8; Y – 2, 8, 7 and Z – 2, 8, 2. Which of the following is correct?
(a) X is a metal
(b) Y is a metal
(c) Z is a non-metal
(d) Y is a non-metal and Z is a metal
Answer: (d) Y is a non-metal and Z is a metal
Explanation: Atomic number of X is 10, this means this is Ne (Neon) which is an inert gas.Atomic number of given element Y is 2 + 8 + 7 = 17. This means this is chlorine (Cl), which is a non–metal.
Atomic number of given element Z is 2 + 8 + 2 = 12. This means this is Magnesium (Mg), which is a metal.
Question: 31. Although metals form basic oxides, which of the following metals form an amphoteric oxide?
(a) Na
(b) Ca
(c) Al
(d) Cu
Answer: (c) Al
Explanation: Aluminium forms amphoteric oxides.
Question: 32. Generally, non-metals are not conductors of electricity. Which of the following is a good conductor of electricity?
(a) Diamond
(b) Graphite
(c) Sulphur
(d) Fullerene
Answer: (b) Graphite
Explanation: Graphite, which is an allotrope of carbon, is only non-metal which conducts electricity.
Question: 33. Electrical wires have a coating of an insulting material. The material, generally used is
(a) Sulphur
(b) Graphite
(c) PVC
(d) All can be used
Answer: (c) PVC
Explanation: PVC is a polymer and bad conductor of electricity. It is used as insulating material for covering electric wires.
Graphite is good conductor of electricity, so cannot be used as insulating material.
Sulphur, which is a non-metal although non-conductor of electricity but brittle in nature. So, cannot be used as insulating material.
Question: 34. Which of the following non-metals is a liquid?
(a) Carbon
(b) Bromine
(c) Phosphorus
(d) Sulphur
Answer: (b) Bromine
Explanation: Metals are generally solid while non-metals are solid, liquid and gas. Bromine is a non-metal and exists as liquid.
Question: 35. Which of the following can undergo a chemical reaction?
(a) MgSO4 + Fe
(b) ZnSO4 + Fe
(c) MgSO4 + Pb
(d) CuSO4 + Fe
Answer: (d) CuSO4 + Fe
Explanation: Iron is more reactive than copper. So, iron displaces copper from copper sulphate solution.
Question: 36. Which one of the following figures correctly describes the process of electrolytic refining?
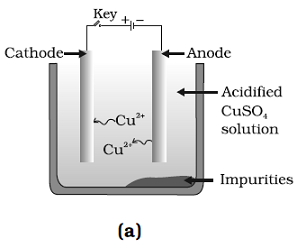
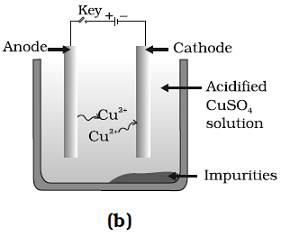
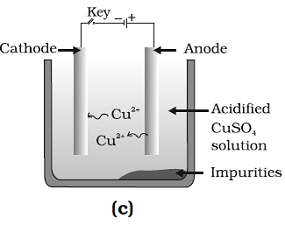
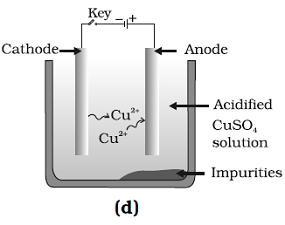
Answer: (c) and (D)
Explanation: A lump of impure copper is taken and connect it to positive pole, and a thin strip of pure metal is taken as cathode. A water soluble metals salt, copper sulphate in the case of copper is taken as electrolyte.
Pure metal move from anode and get deposited at cathode.
Thus, Figure (b) and (c) are correct.