Sample Paper: Term-1:2021-22 - Class 10th Science
Science Term-1 MCQs:A
Question (1) Reema took 5 ml of Barium Chloride solution in a beaker and added approximately 4 ml of Potassium Sulphate solution to it. What would she observe?
(A) The solution turned red.
(B) Yellow precipitate was formed
(C) White precipitate was formed.
(D) The reaction mixture became hot.
Answer (C) White precipitate was formed.
Explanation
When the solution of Potassium Sulphate is mixed with the solution of Barium Chloride, a white precipitate of Barium Sulphate and Potassium Chloride are formed. In this reaction Barium Sulphate is obtained as a white precipitate.
The reaction involved in this can be written as follows:
BaCl2 + K2SO4 ⇒ KCl + BaSO4
This is a double displacement reaction.
Question (2) Identify gas A in the following experiment.
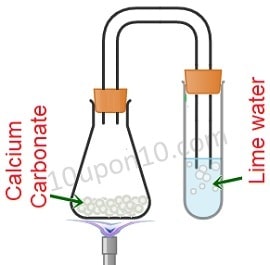
(A) Nitrogen
(B) Hydrogen
(C) Oxygen
(D) Carbon dioxide
Answer : (D) Carbon dioxide
Explanation :
In the given figure, calcium carbonate is being heated. And gas coming from it is going to the lime water.
Calcium carbonate gives carbon dioxide upon heating. Thus the gas is coming out of it is carbon dioxide.
The reaction involves in it can be represented as follows:
CaCO3 + Heat ⇒ CaO + CO2
∴ Option (d) Carbon dioxide is the correct answer.
Question (3) Which of the following metal reacts with steam to produce oxide instead of hydroxide?
(A) Potassium
(B) Calcium
(C) Aluminium
(D) Sodium
Answer (C) Aluminium
Explanation :
When steam is passed over aluminium metal, the aluminium oxide is formed along with hydrogen gas.
2A (s) + 3H2 O (g) ⇒ Al2O3+3H2
Question (4) Which of the following correctly represents a balanced chemical equation?
(A) Fe(S) + 4H2O (g) → Fe3O4 (S) + 4H2(g)
(B) 3Fe(S) + 4H2O (g) → Fe3O4 (S) + 4H2(g)
(C) 3 Fe(S) + 4H2O (g) → Fe3O4 (S) + H2(g)
(D) 3Fe(S) + 4H2O (g) → Fe3O4 (S) + H2(g)
Answer (B) 3Fe(S) + 4H2O (g) → Fe3O4 (S) + 4H2(g)
Explanation
The number of Fe → 3,
H → 8
And, O → 4 on both sides of the equation.
Question (5) On dilution of an acid
(A) The Hydrogen concentration per unit volume increases
(B) The Hydrogen concentration per unit volume decreases
(C) The Hydrogen concentration per unit volume remain same
(D) No impact of dilution on concentration at all.
Answer (B) The Hydrogen concentration per unit volume decreases
Explanation
By dilution of an acid, the number of hydrogen ion remains same while the volume of water increases. So, on dilution of acid, the number of hydrogen ion concentration per unit volume decreases.
Question (6) In the reaction of Zinc with Ferrous Sulphate solution:
FeSO4 + Zn → Fe + ZnSO4
Which option in the given table correctly represents the substance oxidized and the reducing agent?
| Substance | Oxidised | Reducing Agent |
|---|---|---|
| A | Fe | FeSO4 |
| B | Zn | FeSO4 |
| C | Zn | Zn |
| D | ZnSO4 | Zn |
Answer (C) Zn is oxidized and Zn is Reducing Agent
Explanation
Substance which loses electron is called to get oxidized. And substance which loses electrons and get oxidized is called the reducing agent.
In the given reaction, Zn loses electrons and gets oxidized, and hence it is the reducing agent.
Thus, option (c) is the correct answer.
Question (7) The chemical reaction between Magnesium and Oxygen can be categorized as:
(A) Displacement reaction
(B) Decomposition reaction
(C) Combination reaction
(D) Double displacement reaction
Answer (C) Combination reaction
Explanation
When magnesium reacts with oxygen, magnesium oxide is formed.
Mg + O2 → Mgo
In this reaction magnesium is combined with oxygen. Since, magnesium is combined with oxygen, so it is an example of Combination Reaction.
Thus, option (C) Combination Reaction is the correct answer.
Question (8) Which of the given option correctly represents the parent acid and base of Magnesium phosphate?
| Option | Parent Acid | Parent Base |
|---|---|---|
| A | HCl | NaOH |
| B | HNO3 | Mg(OH)2 |
| C | H3PO4 | Mg(OH)2 |
| D | H2SO4 | Mg(OH)2 |
Answer (C) Acid: H3PO4 and Base: Mg(OH)2
Explanation
When Phosphoric acid (H3PO4) reacts with Magnesium hydroxide (Mg(OH)2), it gives magnesium phosphate and water.
Mg3(PO4)2 ⇒ + 3 mg (oH) 2 → (PO4) 2 + 6H2O
Question (9) How will you protect yourself from the heat generated while diluting a concentrated acid?
(A) By adding acid to water with constant stirring.
(B) By adding water to acid with constant stirring.
(C) By adding water to acid followed by base.
(D) By adding base to water with constant stirring.
Answer (A) By adding acid to water with constant stirring.
Explanation
Dilution of acid is an exothermic process this means heat is evolved while dilution of acid.
Thus, if water will be added to acid, because of evolution of heat acid may splashes out and poured on skin resulting in burning of skin.
So, to prevent it, in the course of dilution, acid is added to water drop by drop with constant stirring.
Thus, option (B) By adding water to acid with constant stirring is the correct acid.
Question (10) Why is it important to balance a skeletal chemical equation?
(A) To verify law of conservation of energy.
(B) To verify the law of constant proportion.
(C) To verify the law of conservation of mass.
(B) To verify the law of conservation of momentum.
Answer (C) To verify the law of conservation of mass.
Explanation
The law of conservation of mass states mass can neither be created nor destroyed in a chemical reaction. This means mass of reactants is equal to the mass of products in a reaction.
Thus, in order to obey the law of conservation of mass, a chemical equation is needed to balance.
Thus, option (C) To verify the law of conservation of mass is the correct answer.