Sample Paper: Term-1:2021-22 - Class 10th Science
Science Term-1 MCQs:C
Question (21) At what distance from a concave lens of focal length 20 cm, a 6 cm tall object be placed so as to its image at 15 cm from the lens?
(A) 12 cm
(B) – 12 cm
(C) – 20 cm
(D) – 60 cm
Answer (D) – 60 cm
Explanation
Here, given, f = – 20 cm
Height of the object (μ) = 6 cm
v = – 15 cm
∴ u = ?
We know from the lens formula,
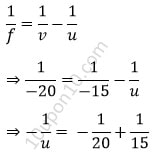
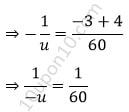
⇒ u = – 60
Thus, object should be placed at 60 cm from the pole.
∴ Option (D) –60 cm is the correct answer.
Question (22) If a refractive index of a medium is μ and the velocity of a light in free space is c, then the velocity of light of that medium is
(A) μC
(B) (c/μ)1/2
(C) (μc) 1/2
(D) c/μ
Answer (D) c/μ
Explanation
Given refractive index = μ
Speed of light in free space = C
Now, we know that, refractive index = (speed of light in vacuum)/(speed of light in the medium)
⇒ μ = c/v
⇒ vμ = c
⇒ v = c/μ
∴ velocity of light in the given medium = c/μ
Hence, option (D) c/μ is the correct answer.
Question (23) The angle of refraction for a ray of light ray which falls perpendicular to the interface of two media is:
(A) 180 degrees
(B) 90 degrees
(C) 45 degrees
(D) 0 degrees
Answer (D) 0 degrees
Explanation
When a light ray falls perpendicular to the interface of media i.e. along the normal, the ray of light does not show any bending.
∴ angle of refraction = 0 degree.
Thus, option (D) 0o is the correct option.
Question (24) A student conducts an activity using a concave mirror with focal length of 10 cm. He placed the object 15 cm from the mirror. Where is the image likely to from?
(A) at 6 cm behind the mirror
(B) at 30 cm behind the mirror
(C) at 6 cm in front of the mirror
(D) at 30 cm in front of the mirror
Answer (D) at 30 cm in front of the mirror
Explanation
Given, focal length (f) = – 10
Distance of object from mirror (u) = – 15cm
Therefore, distance of image (v) = ?
From mirror lens formula, we know that
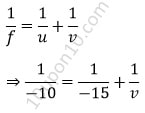
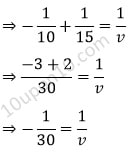
⇒ v = – 30
Since, the distance of image is in minus, so it will form in the front of mirror. And as given focal length is 10. Thus, image will form at 30 cm in front of mirror and beyond C.
Thus, option (D) At 30 cm in front of the mirror is the correct answer.
Question (25) Which of the following statements is correct about an aqueous solution of an acid and of a base?
(i) Higher the pH, stronger the acid
(ii) Higher the pH, weaker the acid
(iii) Lower the pH, stronger the base
(iv) Lower the pH, weaker the base
(A) (i) and (iii)
(B) (ii) and (iii)
(C) (i) and (iv)
(D) (ii) and (iv)
Answer (A) (ii) and (iv)
Explanation
pH scale is worked from 0 (zero) to 14.
The pH value of distilled water is equal to 7.
The strongest base has pH value equal to 14 and weakest base has pH value greater than 7.
This means with the increase in pH value from 7 to 14, the strength of base increases.
On the other hand, the pH value of strongest acid is equal to 1. And the weakest acid has value of pH just below the 7.
This means the strength of acid increases with decrease in value of pH from 7 and downward.
Thus, option (ii) Higher the pH, weaker the acid and (iv) Lower the pH, weaker the base will be the right answer.
Thus, option (D) (ii) and (iv) is the correct answer.
Question (26) Solid calcium oxide reacts vigorously with water to form calcium hydroxide accompanied by liberation of heat. This process is called slaking of lime. Calcium hydroxide dissolves in water to form its solution called lime water. Which among the following is are true about slaking of lime and the solution formed?
(a) It is an endothermic reaction.
(b) it is an exothermic reaction.
(c) The pH of the resulting solution will be more than seven.
(d) The pH of the resulting solution will be less than seven.
(A) (a) and (b)
(B) (b) and (c)
(C) (a) and (d)
(D) (c) and (d)
Answer (B) (b) and (c)
Explanation
Heat is evolved when calcium oxide reacts with water. The reaction in which heat is evolved is called exothermic reaction.
When calcium oxide reacts with water, calcium hydroxide is formed along with evolution of heat.
CaO + H2O ⇒ CaOH2 + Heat
Calcium hydroxide is a base. And the pH value of a base is greater than 7.
Thus, option (b) it is an exothermic reaction and (c) the pH of the resulting solution will be more than seven are the correct answer.
Thus, option (B) (b) and (c) is the correct answer.
Question (27) At ordinary temperature, the surface of metals such as magnesium, aluminum, Zinc, lead, etc. are covered with a thin layer of
(A) Carbonate
(B) Oxide
(C) Sulphide
(D) Basic Carbonate
Answer (B) Oxide
Explanation
Metals react with oxygen present in air and form respective oxides. Formation of this oxide gets deposited on the surface of metals in the form of a thin layer.
Thus, option (B) Oxide is the correct answer.
Question (28) table shown below gives information about some ionic substances
| Ionic compounds | Melting point (K) | Boiling point (K) |
|---|---|---|
| NaCl | 1074 | 1656 |
| LiCl | 897 | 1600 |
| CaCl2 | 1045 | 1000 |
| CaO | 2850 | 3120 |
| MgCl2 | 981 | 1685 |
Which statement is incorrect about ionic compounds
(A) Ionic compounds are generally soluble in water and insoluble in solvents such as kerosene, petrol, etc.
(B) Ionic compounds have high melting and boiling point as less amount of energy is needed to break the force of attraction between them.
(C) Ionic compounds are solids
(D) Ionic compounds are good conductors of electricity
Answer (B) Ionic compounds have high melting and boiling point as less amount of energy is needed to break the force of attraction between them.
Explanation
Ionic compounds need very high amount of energy to break the strong force of attraction of bonding found in them. Because of this ionic compounds have high melting and boiling points.
So, the statement given in option (B) Ionic compounds have high melting and boiling point as less amount of energy is needed to break the force of attraction between them is not correct.
Thus, the correct answer is, option (B) Ionic compounds have high melting and boiling point as less amount of energy is needed to break the force of attraction between them.
Question (29) Vinay observed that the stain of curry on a white shirt becomes reddish-brown when soap is scrubbed on it, but it turns yellow again when the shirt is washed with plenty of water. What might be the reason for his observation?
(i) Soap is acidic in nature
(ii) Soap is basic in nature
(iii) Turmeric is a natural indicator which gives reddish tinge in bases
(iv) Turmeric is a natural indicator which gives reddish tinge in acids
(A) i and ii
(B) ii and iii
(C) i and iv
(D) ii and iv
Answer (B) ii and iii
Explanation
Turmeric is a natural indicator. This turns base in reddish brown colour. And soap is a base.
Thus, when soap is applied over the stain curry on a cloth which contains turmeric, the stain turns reddish brown.
And when this stain is washed with plenty of water, the soap is washed out, and the colour of stain of turmeric becomes yellow.
Thus, the correct answer is option (ii) Soap is basic in nature and option (iii) Turmeric is a natural indicator which gives reddish tinge in bases
Thus, the correct answer is option (B) ii and iii
Question (30) An element X with atomic number 12 combines with Element Y with atomic number 17 identify the formula of the compound formed and its nature
(A) XY and Ionic
(B) X2Y and Electrovalent
(C) XY2 and Ionic
(D) 2XY and Non Ionic
Answer (C) XY2 and Ionic
Explanation
Given, atomic number of element X = 12
And the atomic number of element Y = 17
Thus, formula of the compound formed by these two elements and its nature = ?
We know that Mg (Magnesium) has atomic number equal to 12
And Cl (Chlorine) has atomic number equal to 17
Thus, compound is magnesium chloride. And formula of magnesium chloride is MgCl2
In MgCl2 bonds are formed because of transfer of electrons.
And bond formed because of transfer of electrons is called electrovalent bond and such compounds are known as electrovalent compound.
Thus, it is an electrovalent compound.
Thus, the correct answer is, option (B) X2Y and Electrovalent