Acid Base & Salt - Class 10th Science
NCERT Exemplar Solution:MCQs1
Question: 1. What happens when a solution of an acid is mixed with a solution of a base in a test tube?
- The temperature of the solution increases
- The temperature of the solution decreases
- The temperature of the solution remains the same
- Salt formation takes place
- (i) only
- (i) and (iii)
- (ii) and (iii)
- (i) and (iv)
Answer : (d) (i) and (iv)
Question: 2. An aqueous solution turns red litmus solution blue. Excess addition of which of the following solution would reverse the change?
- Baking powder
- Lime
- Ammonium hydroxide solution
- Hydrochloric acid
Answer: (d) Hydrochloric acid
Explanation: Since, given aqueous solution is basic because it changes red litmus solution blue. When excess of hydrochloric acid is added, the solution becomes acidic.
Question: 3. During the preparation of hydrogen chloride gas on a humid day, the gas is usually passed through the guard tube containing calcium chloride. The role of calcium chloride taken in the guard tube is to
- absorb the evolved gas
- moisten the gas
- absorb moisture from the gas
- absorb Cl— ions from the evolved gas
Answer: (c) absorb moisture from the gas
Question: 4. Which of the following salts does not contain water of crystallisation?
- Blue vitriol
- Baking soda
- Washing soda
- Gypsum
Answer: (b) Baking soda
Question: 5. Sodium carbonate is a basic salt because it is a salt of
- strong acid and strong base
- weak acid and weak base
- strong acid and weak base
- weak acid and strong base
Answer: (d) Weak acid and strong base
Explanation: Sodium carbonate is formed by the reaction between Carbonic acid and sodium hydroxide. Sodium hydroxide is a strong base while carbonic acid is a weak acid. In this reaction carbonic acid does not neutralize sodium hydroxide completely resulting salt formed is basic in nature.

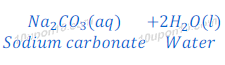
Question: 6. Calcium phosphate is present in tooth enamel. Its nature is
- basic
- acidic
- neutral
- amphoteric
Answer: (c) basic
Explanation: Calcium phosphate is a salt formed by the reaction between phosphoric acid and calcium hydroxide. Phosphoric acid is a weaker acid. Although calcium hydroxide is also weaker base, but slightly stronger than phosphoric acid. Because of this phosphoric acid does not neutralize calcium hydroxide completely. Hence salt (calcium phosphate) formed is slightly basic in nature.
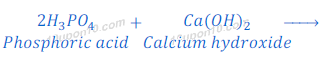
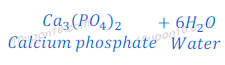
Question: 7. A sample of soil is mixed with water and allowed to settle. The clear supernatant solution turns the pH paper yellowish-orange. Which of the following would change the colour of this pH paper to greenish-blue?
- Lemon juice
- Vinegar
- Common salt
- An antacid
Answer: (d) An antacid
Explanation: Yellowish orange color of pH paper shows the pH value is about 2 to 3. This means soil is highly acidic. And greenish blue color of pH paper shows the pH value is about between 4 and 5.
Thus, to neutralize this acid upto some extent, i.e. to increase pH value near between 4 to 5, an antacid is needed.
Thus, answer is (d) An antacid
Question: 8. Which of the following gives the correct increasing order of acidic strength?
- Water
- Water
- Acetic acid
- Hydrochloric acid
Answer: (a) Water < Acetic acid < Hydrochloric acid
Explanation: pH value of water is abou 7. Acetic acid is a weaker acid while hydrochloric acid is a strong acid.
Thus, the correct increasing order of acidic strength is (a) Water < Acetic acid < Hydrochloric acid
Question: 9. If a few drops of a concentrated acid accidentally spills over the hand of a student, what should be done?
- Wash the hand with saline solution
- Wash the hand immediately with plenty of water and apply a paste of sodium hydrogencarbonate
- After washing with plenty of water apply solution of sodium hydroxide on the hand
- Neutralise the acid with a strong alkali
Answer: (b) Wash the hand immediately with plenty of water and apply a paste of sodium hydrogen carbonate.
Explanation: By washing the hand with plenty of water base will washed away. And applying sodium hydrogen carbonate, which is a mild base, will neutralize the effect of base if any leftover hand.
Question: 10. Sodium hydrogencarbonate when added to acetic acid evolves a gas. Which of the following statements are true about the gas evolved?
- It turns lime water milky
- It extinguishes a burning splinter
- It dissolves in a solution of sodium hydroxide
- It has a pungent odour
- (i) and (ii)
- (i), (ii) and (iii)
- (ii), (iii) and (iv)
- (i) and (iv)
Answer: (a) (i) and (ii)
Explanation: Reaction of sodium hydrogen carbonate and acetic acid gives carbon dioxide gas. Carbon dioxide gas turns lime water milky and extinguishes fire.
Thus, option (i) and (ii) are correct. And answer (a) (i) and (ii)