Acid Base & Salt - Class 10th Science
Baking Soda (Sodium Bicarbonate)
Baking soda is another chemical obtained from sodium chloride. The chemical name of baking soda is sodium bicarbonate or sodium hydrogen carbonate (NaHCO3). Other name of baking soda is bread soda, cooking soda, bicarbonate of soda, sodium bicarb, bicarb of soda and bicarb, etc.
Baking soda is used in baking of food and making of food tasty. It is also used in faster cooking.
Production of baking soda (sodium bicarbonate):
(a) By Solvay Process: Baking soda (Sodium bicarbonate) is produced by reaction of sodium chloride, carbon dioxide and ammonia. This process is known as Solvay Process.
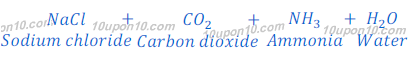

(b) When carbon dioxide is passed through the solution of sodium hydroxide, sodium carbonate is produced. After passing more carbon dioxide, this sodium carbonate (baking soda) changes into sodium bicarbonate.
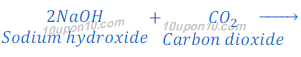
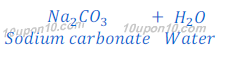
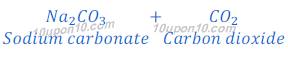
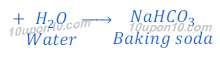
Properties of sodium bicarbonate:
Sodium bicarbonate is amphoteric in nature. An amphoteric compound reacts in similar way with an acid and a base both.
Sodium bicarbonate is a white solid. Sodium bicarbonate appears as powder but actually it is crystalline.
Sodium bicarbonate is sparingly soluble in water.
Sodium bicarbonate when dissolved in water, gives carbonic acid and hydroxide ion. Since carbonic acid is weak acid, thus solution of sodium bicarbonate is alkaline in nature.
When sodium bicarbonate (baking soda) put under thermal decomposition it gives sodium carbonate, carbon dioxide and water.

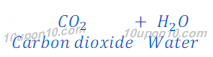
On continuous heating sodium carbonate produced in thermal decomposition of sodium bicarbonate converts into sodium oxide and carbon dioxide. This reaction is called dehydration reaction.
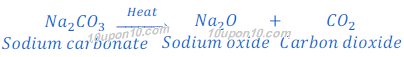
As it gives carbon dioxide, which put off fire, sodium bicarbonate is used as fire extinguisher or fire suppression agent. It is also known as BC powder.
Some Uses of sodium bicarbonate (Baking soda):
- Baking soda is used in cooking. Baking soda on heating produced carbon dioxide which makes barter soft and spongy.
- Sodium bicarbonate is a base thus it is used as antacid.
- Sodium bicarbonate is also used in the treatment of overdose of aspirin.
- Mixing of sodium bicarbonate in water is exothermic. This character makes it an antiseptic.
- It is used in toothpaste. This whitening the teeth and remove plaque.
- Sodium bicarbonate is also used as cleaning and scrubbing.
- It is used as bio-pesticide.
- Sodium bicarbonate is used in cattle feed as it acts as buffering agent for rumen.
- Sodium bicarbonate is used as fire extinguisher for mild fire.
- In fire extinguisher it is used as one of the component. In fire extinguisher sodium bicarbonate is allowed to react with sulphuric acid kept in a separate chamber, in the condition of fire. Reaction of sodium bicarbonate and sulphuric acid gives carbon dioxide. Carbon dioxide so produced is thrown over the fire, which cover the fire and stop the supply of oxygen. This process put off the fire. This fire extinguisher is also known as soda-acid fire extinguisher.