Acid Base & Salt - Class 10th Science
Washing Soda (Sodium Carbonate)
Washing soda is another important chemical obtained from sodium chloride (common salt). The chemical name of washing soda is sodium carbonate (Na2CO3). Washing soda is the sodium salt of carbonic acid. As its name says, washing soda is used in washing.
Production of washing soda:
By Solvay Process:
In Solvay process first sodium bicarbonate is produced. This sodium bicarbonate on heating gives sodium carbonate (washing soda).
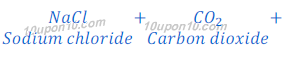
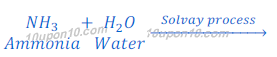
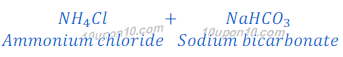

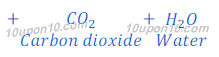
Sodium carbonate obtained in this Solvay process is dry, so it is also called soda ash.
The Solvay process got its name after Ernest Solvay who developed this process in 1861. Earnest Solvay was an industrial chemist and belonged to Belgian.
Soda ash (anhydrous soda) to washing powder:
By treating with water anhydrous sodium carbonate (soda ash) is converted into washing soda.

Since, washing power contains 10 (ten) water molecules, thus is called sodium carbonate decahydrate.
Properties of washing soda:
- Sodium carbonate is a crystalline transparent solid.
- Where most of the carbonates are insoluble in water, sodium carbonate is soluble in water.
Some Uses of sodium carbonate (Washing soda):