Acid Base & Salt - Class 10th Science
NCERT InText Solution:1
Question: 1: You have been provided with three test tubes. One of them contains distilled water and the other two contain an acidic solution and a basic solution, respectively. If you are given only red litmus paper, how will you identify the contents of each test tube?
Answer: An acid solution turns red litmus paper blue and a basic solution turns blue litmus paper red. While distilled water does not act upon litmus paper.
Step: 1: Solution in test tube which changes red litmus paper blue is acid.
Step: 2: Now, blue litmus paper which is changed blue by acid is dipped in rest of the two test tubes one by one. Solution which changes the blue litmus paper red is basic solution.
Step: 3: Third one solution in test tube left, which does not change either red litmus blue or blue litmus paper red is identified as distilled water.
Question: 2: Why should curd and sour substances not be kept in brass and copper vessels?
Answer: Metals on reaction with acid produces hydrogen gas and respective salt. Copper is a metal and brass is an alloy of copper metal. Thus, copper and brass reacts with acid and form hydrogen gas.
Curd and other sour substances contain acid. That’s why curd and other sour substances are not kept in the brass and copper vessels.
Question: 3: Which gas is usually liberated when an acid reacts with a metal? Illustrate with an example. How will you test for the presence of this gas?
Answer: When metal reacts with acid, it produces hydrogen gas.
Example:
When zinc metal reacts with hydrochloric acid, it produces hydrogen gas and zinc chloride.
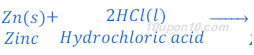
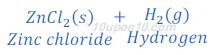
When a burning candle or match stick is brought near the gas evolved, it burns with pop sound. Burning with pop sound proves the presence of hydrogen gas. Burning with pop sound is the characteristic test for hydrogen gas.
Question: 4: Metal compound A reacts with dilute hydrochloric acid to produce effervescence. The gas evolved extinguishes a burning candle. Write a balanced chemical equation for the reaction if one of the compounds formed is calcium chloride.
Answer: Calcium carbonate produces carbon dioxide gas with effervescence along with calcium chloride when reacts with dilute hydrochloric acid.
Since, carbon dioxide gas is used as fire extinguisher therefore, the given metal compound A is calcium carbonate.
Balanced chemical equation for above reaction:
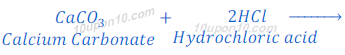
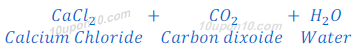
Question: 5: Why do HCl, HNO3, etc., show acidic characters in aqueous solutions while solutions of compounds like alcohol and glucose do not show acidic character?
Answer: it is the presence of hydrogen ions in aqueous solution which is responsible for acidic character of a solution.
HCl, HNO3, etc., dissociate hydrogen ions in their aqueous solutions. And because of dissociation of hydrogen ions HCl, HCl, HNO3, etc. shows acidic characters.
While compounds like alcohol and glucose do not dissociate hydrogen ions in their aqueous solutions. Thus, in absence of hydrogen ions compounds like alcohol and glucose do not show acidic characters.
Question: 6: Why does an aqueous solution of an acid conduct electricity?
Answer: It is ions which are responsible for conduction of electricity in solution.
Acid dissociates hydrogen ions and hydroxide ions in their aqueous solution, thus because of presence of ions acid conducts electricity in their aqueous solutions.
Question: 7: Why does dry HCl gas not change the colour of the dry litmus paper?
Answer: It is the hydrogen ions or hydronium ions which is responsible for acidic character of a substance.
Dry HCl gas or any acid does not dissociate hydrogen ions in absence of water. That’s why dry HCl gas does not change the colour of dry litmus paper.
But, if litmus paper is moist, dry HCl gas changes the color of blue litmus paper red.
Question: 8: While diluting an acid, why is it recommended that the acid should be added to water and not water to the acid?
Answer: Dilution of acid or a base is highly exothermic. If water is added to concentrated acid for dilution, because of presence of acid in large amount, large amount of heat is evolved which will be dangerous as acid may splashes out and burn the skin.
Thus, it is recommended that always acid is added to water slowly and not water to acid.