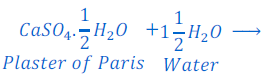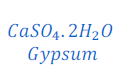Acid Base & Salt - Class 10th Science
NCERT InText Solution:2
Question: 9: How is the concentration of hydronium ions (H3O+) affected when a solution of an acid is diluted?
Answer: The concentration of acid decreases when diluted. Consequently concentration of hydronium ions (H3O+) decreases when an acid is diluted.
Question: 10: How is the concentration of hydroxide ions (OH–) affected when excess base is dissolved in a solution of sodium hydroxide?
Answer: A base dissociates hydroxide ions in its aqueous solution. Thus, when excess base is dissolved in the solution of sodium hydroxide, which is a base, the concentration of hydroxide ions (OH–) will increase.
Question: 11: You have two solutions, A and B. The pH of solution A is 6 and pH of solution B is 8. Which solution has more hydrogen ion concentration? Which of this is acidic and which one is basic?
Answer: Hydrogen ions concentration increases with decrease in the value of pH.
Solution A has pH value equal to 6 and solution B has pH value equal to 8. Since, solution A has less pH value consequently solution A has more hydrogen ions concentration.
An acid has pH value less than 7 while a base has pH value more than 7. Since solution A has pH value less than 7, i.e. 6 thus, solution A is acidic.
And solution B has pH value more than 7, i.e. 8, thus solution B is basic.
Question: 12: What effect does the concentration of H+(aq) ions have on the nature of solution?
Answer: (1) Hydrogen ions concentration decides the acidic or basic nature of solution. The acidity of solution increases with increase in hydrogen ions concentration and decreases with decrease in hydrogen ions concentration.
Answer: (2) Nature of solution may be of two types:
(a) Concentrated or diluted
(b) Acidic or basic
(a) Concentrated or diluted:
A concentrated acid solution has more concentration of H+(aq) ions compare to diluted acid solution.
(b) Acidic or Basic
An acidic solution has more concentration of H+(aq) ions compare to hydroxide ions. While a basic solution has less concentration of H+(aq) ions compare to hydroxide ions concentration.
Question: 13: Do basic solution also have H+(aq) ions? If yes, then why are these base?
Answer: Yes basic solution also have H+(aq) ions.
But a base has less hydrogen ions concentration than hydroxide ions. It is the hydroxide ions concentration that decides that whether a solution is base or not.
Thus, solution which has also hydrogen ions but in less amount than hydroxide ions, are base.
Question: 14: Under what soil condition do you think a farmer would treat the soil of his fields with quick lime (calcium oxide) or slaked lime (calcium hydroxide) or chalk (calcium carbonate)?
Answer: Quick lime (calcium oxide) or slaked lime (calcium hydroxide) or chalk (calcium carbonate) are base which neutralizes acids.
Thus, if the soil condition is acidic, farmer would treat the soil of his field with quick lime (calcium oxide) or slaked lime (calcium hydroxide) or chalk (calcium carbonate) to neutralize the excess acid present in soil.
Question: 15: What is the common name of the compound CaOCl2?
Answer: The Common name of the compound CaOCl2 is bleaching powder.
Question: 16: Name the substance which on treatment with chlorine yields bleaching powder.
Answer: When calcium hydroxide [Ca(OH)2] is treated with chlorine, it yields bleaching powder (CaOCl2).
Question: 17: Name the sodium compound which is used for softening hard water.
Answer: Sodium carbonate [Washing soda (Na2CO3)] is used for softening hard water.
Question: 18: What will happen if a solution of sodium hydrocarbonate is heated? Give the equation of the reaction involved.
Answer: When solution of sodium hydrocarbonate is heated, it produces sodium carbonate, water and carbon dioxide gas is formed.

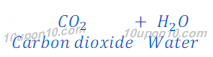
Question: 19: Write an equation to show the reaction between Plaster of Paris and water.
Answer: