Acid Base & Salt - Class 10th Science
NCERT Exercise solution:1
Question: 1: A solution turns red litmus blue. It pH is likely to be
- 1
- 4
- 5
- 10
Answer: (d) 10
Explanation: A base turns red litmus blue. Solution having pH value more than 7 are base. Thus, here answer is (d) with pH value 10
Question: 2: A solution reacts with crushed egg-shells to give a gas that turns lime-water milky. The solution contains
- NaCl
- HCl
- LiCl
- KCl
Answer: (b) HCl
Explanation: egg shells are made of calcium carbonate. When calcium carbonate reacts with hydrochloric acid, it produces carbon dioxide gas. When this carbon dioxide gas is passed through lime water, lime water turns milky.
Thus, here answer is (b) HCl
Question: 3: 10 mL of a solution of NaOH is found to be completely neutralized by 8 mL of a given solution of HCl. If we take 20 mL of the same solution of NaOH, the amount of HCl solution (the same solution as before) required to neutralize it will be
- 4mL
- 8mL
- 12mL
- 16mL
Answer: (d) 16mL
Explanation: Since 10 mL of NaOH solution requires 8 mL of HCl solution
Therefore, 20 mL of NaOH solution will require 8 x 2 = 16 mL of HCl solution
Question: 4: Which one of the following types of medicines is used for treating indigestion?
- Antibiotic
- Analgesic
- Antacid
- Antiseptic
Answer: (c) Antacid
Explanation: Because of over eating our stomach produce more acid, which is generally resulting in indigestion. Thus, to neutralize excess acid produced by stomach antacid is taken as medicine. Thus answer is (c) Antacid
Question: 5: Write word equations and them balanced equations for the reaction taking place when
(a) dilute sulphuric acid reacts with zinc granules
Answer:
Sulphuric acid + Zn → Zinc sulphate + Hydrogen
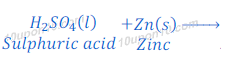
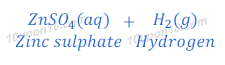
(b) dilute hydrochloric acid reacts with magnesium ribbon
Answer:
Hydrochloric acid + Magnesium → Magnesium chloride + Hydrogen
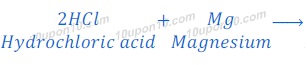

(c) dilute sulphuric acid reacts with aluminium powder
Answer:
Sulphuric acid + Aluminium → Aluminium sulphate + Hydrogen

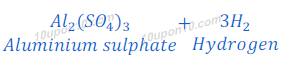
(d) dilute hydrochloric acid reacts with iron fillings.
Answer:
Hydrochloric acid + Fe → Iron chloride + Hydrogen
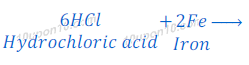
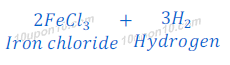
Question: 6: Compounds such as alcohols and glucose also contain hydrogen but are not categorized as acid. Describe an activity to prove it.
Answer:
A cork is taken and got fitted with two iron nails and connected to battery, bulb and switch.
Cork is placed in the beaker
Alcohol is poured in beaker in such a manner that half of the portion of nails is dipped in it.
Now switch is put on
Bulb is observed that whether it glows or not
This activity is repeated with glucose and hydrochloric acid solution
It is observed that bulb does not glow in the case of alcohol and glucose, but bulb glows in the case of hydrochloric acid solution.
Hydrogen ions are responsible for conduction of electricity in the case of acid. Since hydrochloric acid dissociates hydrogen ions in its aqueous solution, thus electricity conducts through the aqueous solution of hydrochloric acid. But aqueous solution of glucose and alcohol do not dissociate hydrogen ions in their aqueous solution, that’s why these solution do not conduct electricity and bulb does not glow.
This activity proves that alcohols and glucose are not acid instead they contain hydrogen because these do not dissociate hydrogen ions in their aqueous solution.