Acid Base & Salt - Class 10th Science
NCERT Exemplar Solution:Long Answer
Question: 43. In the following schematic diagram for the preparation of hydrogen gas as shown in Figure 2.3, what would happen if following changes are made?
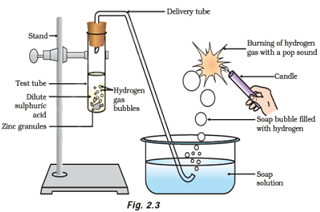
(a) In place of zinc granules, same amount of zinc dust is taken in the test tube
(b) Instead of dilute sulphuric acid, dilute hydrochloric acid is taken
(c) In place of zinc, copper turnings are taken
(d) Sodium hydroxide is taken in place of dilute sulphuric acid and the tube is heated.
Answer:
(a) In the case of zinc dust instead of zinc granules, hydrogen gas is formed.
When zinc dust reacts with sulphuric acid hydrogen gas and zinc sulphate are formed.
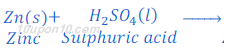
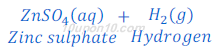
(b) If dilute hydrochloric acid is taken instead of sulphuric acid same result will form, i.e. hydrogen gas evolves.
When zinc granules react with dilute hydrochloric acid, it gives zinc chloride and hydrogen gas.
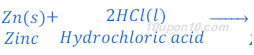
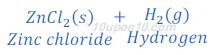
(c) If copper turning are taken at the place of zinc granules, in this case also hydrogen gas is formed.
When copper turning reacts with dilute sulphuric acid, copper sulphate and hydrogen gas are formed.
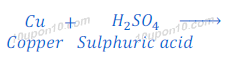
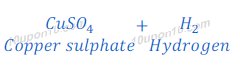
(d) If sodium hydroxide is taken in place of dilute sulphuric acid and the tube is heated, in this case also hydrogen gas is formed.
When zinc is heated with sodium hydroxide, sodium zincate and hydrogen gas are formed.
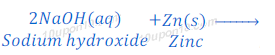
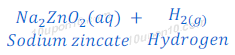
Question: 44. For making cake, baking powder is taken. If at home your mother uses baking soda instead of baking powder in cake,
(a) how will it affect the taste of the cake and why?
(b) how can baking soda be converted into baking powder?
(c) what is the role of tartaric acid added to baking soda?
Answer:
(a) When baking soda is heated, it gives carbon dioxide, water and sodium carbonate.

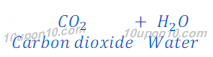
Thus, if baking soda is used in making of cake, formation of sodium carbonate makes the taste of cake bitter. Because sodium carbonate is a base and a base is bitter in taste.
On the other hand, baking powder on heating does not forms sodium carbonate, and hence does not make the taste of cake bitter.
(b) Baking powder is a mixture of baking soda (sodium bicarbonate) and tartaric acid or other edible acid.
Baking powder is made by mixing of baking soda and tartaric acid or other mild edible acid.
(c) There are two roles of baking powder in making of cake:
(i) When water is added to the baking powder, tartaric acid present in baking powder dissociates hydrogen ions. These hydrogen ions react with sodium bicarbonate and produce carbon dioxide at room temperature. Carbon dioxides get trapped into dough and come out very slowly in the form of bubbles. These bubbles make the batter of cake soft and spongy.
(ii) On heating of baking powder, sodium carbonate also formed. This sodium carbonate reacts with acid present in baking powder and produces sodium tartarate and water.
Taste and smell of sodium tartarate is pleasant. This gives pleasant smell and taste to cake.
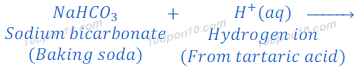
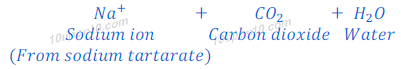
Question: 45. A metal carbonate X on reacting with an acid gives a gas which when passed through a solution Y gives the carbonate back.
On the other hand, a gas G that is obtained at anode during electrolysis of brine is passed on dry Y, it gives a compound Z, used for disinfecting drinking water. Identity X, Y, G and Z.
Answer:
Part (A)
When calcium carbonate reacts with hydrochloric acid, carbon dioxide gas, water and calcium chloride are formed.
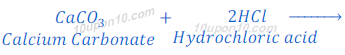
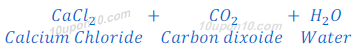
When the carbon dioxide is passed through the lime water, lime water turns milky because of the formation of calcium carbonate.
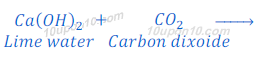
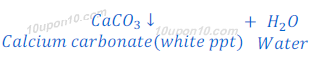
Therefore,
Metal carbonate ‘X’ is calcium carbonate.
Solution ‘Y’ is lime water (Calcium hydroxide).
Part (B)
When electric current is passed through brine (solution of sodium chloride), chlorine gas is deposited near anode. When chlorine gas is passed over dry slaked lime (calcium hydroxide), it produces calcium oxychloride (bleaching powder) and with water.
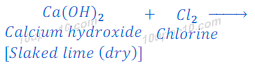
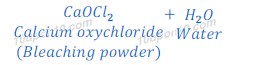
The bleaching powder (calcium oxychloride) is used for disinfecting of drinking water.
Thereore,
Gas ‘G’ is chlorine gas.
Dry ‘Y’ is dry calcium hydroxide (dry slaked lime).
Compound ‘Z‘ is bleaching powder (Calcium oxychloride).