Acid Base & Salt - Class 10th Science
NCERT Exemplar Solution:Long Answer2
Question: 46. A dry pellet of a common base B, when kept in open absorbs moisture and turns sticky. The compound is also a byᾰproduct of chlor—alkali process. Identify B. What type of reaction occurs when B is treated with an acidic oxide? Write a balanced chemical equation for one such solution.
Answer:
(Part—A)
Dry pellets of sodium hydroxide absorb moisture and turn sticky when kept in open. Sodium hydroxide is a byproduct of chlor—alkali process.
Therefore, common base ‘B’ is sodium hydroxide.
(Part—B)
Non-metallic oxides are also known as acidic oxide. For example: carbon dioxide.
Acidic oxides give salt and water when reacts with a base.
Example:
When sodium hydroxide reacts with carbon dioxide (acidic oxide), sodium carbonate and water are formed.
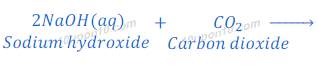
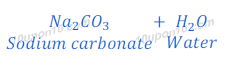
Question: 47. A sulphate salt of Group 2 element of the Periodic Table is a white, soft substance, which can be moulded into different shapes by making its dough. When this compound is left in open for some time, it becomes a solid mass and cannot be used for moulding purposes. Identify the sulphate salt and why does it show such a behaviour? Give the reaction involved.
Answer:
Calcium is a Group 2 element in Periodic Table.
Sulphate salt of calcium is calcium sulphate. The common name of calcium sulphate hemihydrate is Plaster of Paris. The chemical formula is CaSO 4. ½ H2O.
Plaster of Paris is a white soft substance and is used in moulding of clay, toys, desining of wall, etc. after making its dough.
When Plaster of Paris is left in open for some times, it gets hydrated, and turns into Gypsum. The chemical formula of Gypsum is CaSO 4. 2 H2O. This Gypsum again dehydrated after some times and turns into solid mass. After turning into solid mass it cannot be used for moulding purpose.
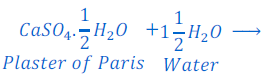
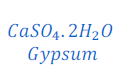
Calcium belongs to group 2 in periodic table, and it’s sulphate salt is Plaster of Paris.
Question: 48. Identify the compound X on the basis of the reactions given below. Also, write the name and chemical formulae of A, B and C.
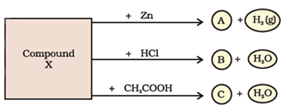
Answer:
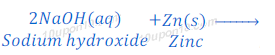
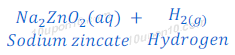

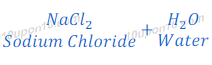
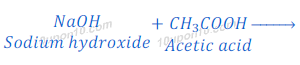
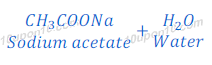
Therefore,
Compound ‘X’ is sodium hydroxide (NaOH).
Compound ‘A’ is Zinc sulpahte (ZnSO4)
Compound ‘B’ is Sodium chloride (NaCl).
Compound ‘C’ is sodium acetate (CH3COONa)