Acid Base & Salt - Class 10th Science
Neutralization Reaction
How do Acids and Bases React with each other?
Acid and base react with each other and produce respective salt and water.
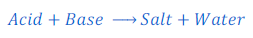
Examples:
Reaction of hydrochloric acid with base:
When hydrochloric acid reacts with sodium hydroxide, sodium chloride and water are formed.
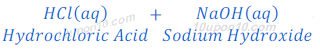
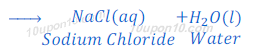
When hydrochloric acid reacts with calcium hydroxide, calcium chloride and water are produced.
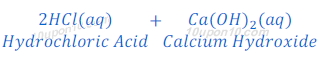
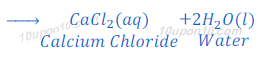
Reaction of Sulphuric Acid with Base:
When sulphuric acid reacts with sodium hydroxide, it produces sodium sulphate and water.
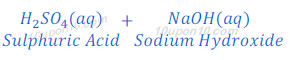
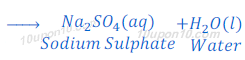
When sulphuric acid reacts with magnesium hydroxide, magnesium sulphate and water are formed.
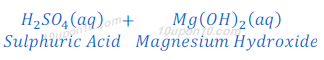
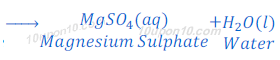
Reaction of nitric acid with base:
When nitric acid reacts with calcium hydroxide, calcium nitrate and water are formed.
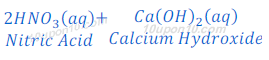
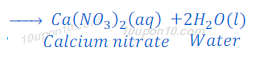
Reaction an acid with a base is called neutralization reaction. Since, in such reactions acid neutralizes the base.
Use of Neutralization Reaction:
Acidity:
Acidity is a medical condition which resulted in cramp in stomach and uneasiness to a person. Acidity is one of the common results of overeating or indigestion. In the case of over-eating our stomach produces more acid to digest the food resulting in the form of acidity.
In the case of acidity doctors give antacid. Antacid is a medicine comes in the form of suspension or tablet. Antacid means anti of acid, i.e. substance which works against acid. Antacid is a base. Generally milk of magnesia (a base) is used as antacid in the condition.
Antacid (base) goes in stomach and neutralizes the excess acid produced. After neutralization of excess acid in our stomach, person suffering from acidity feels relax.
Bee sting:
Honey bee pushes venom through its sting in skin. The venom so injected is acidic. Thus by applying substance containing base or mild base over the stung area gives relief to the victim by neutralizing the effect of acid of venom.
Wasp sting:
Wasp sting is alkaline. Thus by applying lemon, vinegar or other mild acid over the stung area neutralizes the effect of alkali pushed in the form of venom through sting, give relief to the victim person.