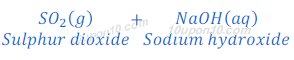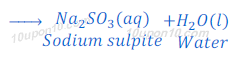Acid Base & Salt - Class 10th Science
Chemical Properties-4
Reaction of Metallic Oxide with Acids
Metal oxides are said to be basic oxides. This means metal oxides are basic in nature. Thus when a metal oxide reacts with acids, respective salt and water are produced.
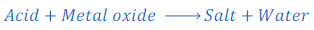
Examples:
(1) When hydrochloric acid reacts with copper oxide, copper chloride and water are formed.
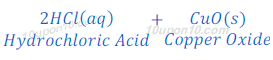
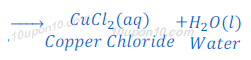
The color of copper oxide is black in color. After reaction with hydrochloric acid, the solution becomes blue green because of formation of copper chloride.
(2) When hydrochloric acid reacts with calcium oxide, calcium chloride and water are formed.
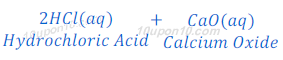
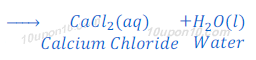
Reaction of a Non-metallic Oxide with Base
Non-metallic oxides are acidic in nature. For example, carbon dioxide is a non-metallic oxide, when carbon dioxide dissolve in water it produces carbonic acid.
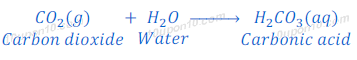
Thus, when a non-metallic oxide reacts with base, it forms respective salt and water.
Non – metallic oxide + Base → Salt + Water
Example:
(1) When carbon dioxide which is a non-metallic oxide, reacts with sodium hydroxide, it gives sodium carbonate and water.
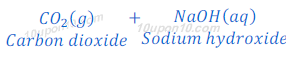
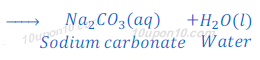
(2) When sulphur dioxide, which is a non-metallic oxide, reacts with sodium hydroxide, it gives sodium sulphite and water.