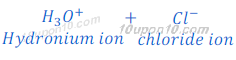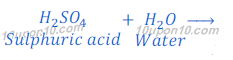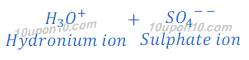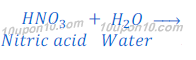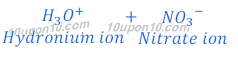Acid Base & Salt - Class 10th Science
Common in Acid and Base
What do all Acids and All Bases have in Common?
Acids when react with metals, produces hydrogen gas. This means it is appears that all acid contains hydrogen. For example: Hydrochloric acid, sulphuric acid, Nitric acid, acetic acid, etc.
But all compounds which contain hydrogen are not acid. For example: glucose, alcohol, etc. While glucose and alcohol contain hydrogen but they are not acid.
On the other hand a base also produces hydrogen gas when reacts with a metal. This also suggests that a base also contains hydrogen.
Thus, it can be concluded that acid and base both contains hydrogen atom in any form.
Acids conduct electricity in aqueous solution
Conduction of electricity in aqueous solution depends upon free ions present in solution. When an acid is dissolved in water, it conducts electricity.
The conduction of electricity through aqueous solution of acids suggests that acids produce hydrogen ions [H+(aq)] in solution. These hydrogen ions are responsible for conduction of electricity through solution.
What happens to an Acid or a Base in Water Solution?
Acid in water solution
When an acid is dissolved in water it gives hydrogen ion [H + (aq)].
Examples:
When hydrochloric acid (HCl) is dissolved in water it gives hydrogen ion (H+) and chloride ion (Cl—).
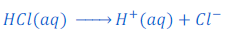
When sulphuric acid (H2SO4) is dissolved in water it gives hydrogen ion (H+) and sulphate ion (SO4— —).
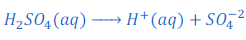
When nitric acid (HNO3) is dissolved in water it gives hydrogen ion (H+) and nitrate ion (NO3—).
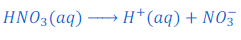
When acetic acid (CH3COOH) is dissolved in water it gives acetate ion (CH3COO—) and hydrogen ion (H+)
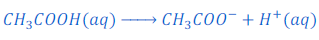
Is an acid produces hydrogen ion in absence of water?
Acid does not dissociate hydrogen ion without water. To dissociate hydrogen ion water is necessary for acid.
This can be proved using an experiment.
When sodium chloride reacts with sulphuric acid, it produces dry hydrochloric acid gas.
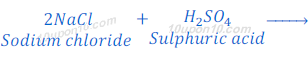
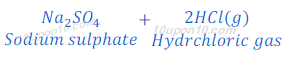
When a dry blue litmus paper is brought near the evolved hydrochloric acid gas no change is observed in the colour of litmus paper. But when a moist litmus paper is brought near the hydrochloric acid gas, litmus paper turns to red.
This suggest that water is necessary to dissociate hydrogen ion [H+(aq)] for an acid.
When an acid is dissolved in water, hydrogen ion (H+) dissociated by acid get combined with water molecule and produced hydronium ion (H3O+). This happens because hydrogen ion cannot exist alone.
The intermediate reaction to give hydrogen ion by hydrochloric acid in water can be shown as follows:
HCl + H2 ⇒ H+ + H2O + Cl— ⇒ H3O+ + Cl—
This may also be written as:
H+ + H2O ⇒ H3O+
Thus, hydrogen ion is always written as H+(aq). After writing the symbol of hydrogen ion the word ‘aq’ is always written in the bracket. This shows the presence of water.
Or hydrogen ion is written as Hydronium ion (H3O+).
Examples: