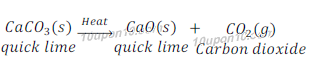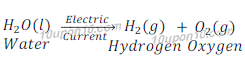Chemical Reactions and Equations - Class 10th Science
NCERT Exercise solution:1
Question: 7: Write the balanced chemical equations for the following reactions
(a) Calcium hydroxide + Carbon dioxide →Calcium carbonate + Water
Answer:
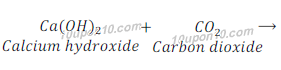
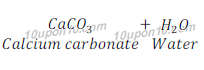
(b) Zinc + Silver nitrate → Zinc nitrate + Silver
Answer:
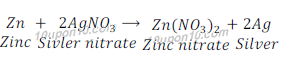
(c) Aluminium + Copper chloride → Aluminium chloride + Copper
Answer:
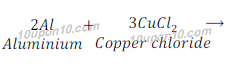
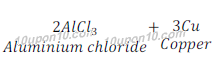
(d) Barium chloride + Potassium sulphate →Barium sulphate + Potassium chloride
Answer:
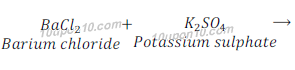
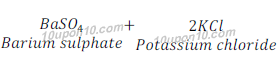
Question: 8: Write the balanced chemical equation for the following and identify the type of reaction in each case.
(a) Potassium bromide (aq) + Barium iodide (aq) → Potassium iodide (aq) + Barium bromide (s)
Answer:
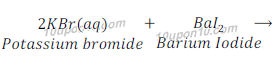
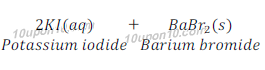
This is double displacement reaction
(b) Zinc carbonate (s) → Zinc oxide (s) + Carbon dioxide (g)
Answer:
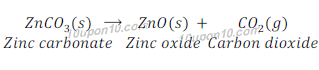
This is a decomposition reaction
(c) Hydrogen (g) + Chlorine (g) → Hydrogen chloride
Answer:
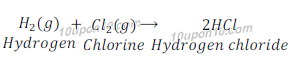
This is a combination reaction
(d) Magnesium (s) + Hydrochloric acid (aq) → Magnesium chloride (aq) + Hydrogen(g)
Answer:
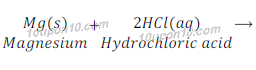
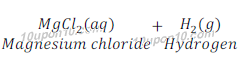
This is a displacement reaction.
Question: 9: What does one mean by exothermic and endothermic reactions? Give examples.
Answer:
Exothermic Reaction
Chemical reactions in which heat is evolved are called Exothermic Reactions.
Example:
When quick lime (Calcium oxide) reacts with water, calcium oxide is formed and heat evolved.
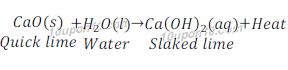
Since, heat evolved in this reaction, thus it is an exothermic reaction.
Endothermic Reaction:
Reactions in which heat is absorbed are called Endothermic Reactions. All of the decomposition reactions require energy in any form, thus all of them are known as endothermic reactions. Thermal decompositions are also known as endothermic reactions.
Example:
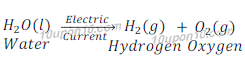
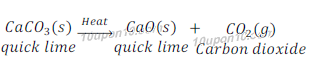
Question: 10: Why is respiration considered an exothermic reaction? Explain.
Answer: We get energy by the process of digestion of food. Digestion of food requires a process called respiration. In respiration glucose is broken into simpler form and produce energy. Since, energy is produced during the process of respiration, thus respiration is considered as an exothermic reaction.
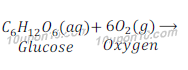
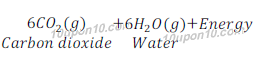
Question: 11: Why are decomposition reactions called the opposite of combination reactions? Write equations for these reactions.
Answer: In combination reaction two or more atoms or molecules combine to form one molecule or compound while in decomposition reaction one compound or molecule is broken into two or more atoms or molecules. Since the process of decomposition reaction is just reverse of combination reaction, that’s why decomposition reactions are called the opposite of combination reactions.
(a) 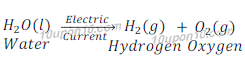
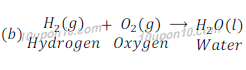
In reaction (a) water decomposes and gives hydrogen and oxygen while in reaction (b) hydrogen and oxygen combines to give water.
Question: 12: Write one equation each for decomposition reactions where energy is supplied in the form of heat, light or electricity.
Answer: