Chemical Reactions and Equations - Class 10th Science
NCERT Exercise solution:2
Question: 13: What is the difference between displacement and double displacement reactions? Write equations for these reactions.
Answer:
In displacement reaction one atom or molecule displaces an atom or molecule from another compound while in double displacement reaction there is exchange of atom, molecule or ions taken place.
Displacement reaction is also called single displacement reaction because in displacement reaction displacement of only one atom or molecule takes place while in double displacement reaction displacement of two atom, molecule or ion is taken place.
Example of displacement reaction:
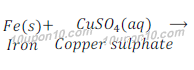
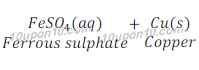
Example of double displacement reaction:
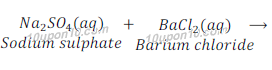
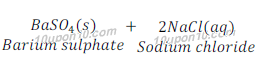
Question: 14: In the refining of silver, the recovery of silver from silver nitrate solution involved displacement by copper metal. Write down the reaction involved.
Answer:
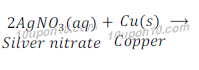
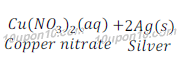
Question: 15: What do you mean by a precipitation reaction? Explain by giving examples.
Answer: Reaction in which precipitate is obtained as one of the product is called precipitation reaction.
Example: When a solution of barium chloride is added to the solution of sodium sulphate, a white precipitate of barium sulphate is obtained along with solution sodium chloride.
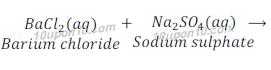
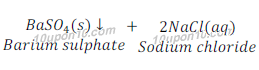
A down arrow is generally put after the compound which is obtained as a precipitate.
Question: 16: Explain the following in terms of gain or loss of oxygen with two examples each.
(a) Oxidation
(b) Reduction
Answer:
(a) Oxidation: When a chemical substance gains oxygen and forms a new compound, the substance is said to be oxidized. This process is called oxidation and the reaction is called an oxidation reaction.
When magnesium ribbon is burnt in the presence of air, magnesium gets oxidized and gives magnesium oxide.
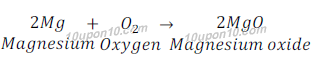
In this reaction, magnesium gains oxygen from the air and hence gets oxidized. This is an oxidation reaction.
When copper is heated in the air, it gets oxidized and gives copper oxide.
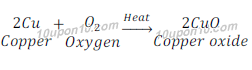
In this reaction copper gains oxygen, and hence copper becomes oxidized. The reaction is called an oxidation reaction.
(b) Reduction: When a chemical substance loses oxygen and forms a new compound, the substance is said to be reduced. The reaction is called oxidation reaction and the process is called reduction.
Example:1: When copper oxide is heated with hydrogen, it loses oxygen and is reduced to copper.
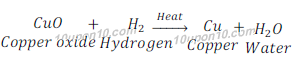
In this reaction, copper oxide loses oxygen and hence is reduced to copper.
Example: 2: When Zinc oxide reacts with carbon, it gives zinc and carbon monoxide.
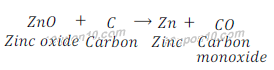
In this reaction zinc oxide is reduced to zinc after losing oxygen.
Question: 17: A shiny brown coloured element ‘X’ on heating in air becomes black in colour. Name the element ‘X’ and black coloured compound formed.
Answer: The sniny brown coloured element ‘X’ is copper.
When copper is heated in the air, it gets oxidized and gives copper oxide.
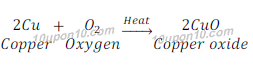
The colour of copper oxide is black in colour.
Question: 18: Why do we apply pain on iron articles?
Answer: Iron articles when exposed to moisture present in the air a layer of iron oxide forms over them. Iron oxide is brown in colour and commonly known as rust. Rust gradually degrades and finally destroys the iron articles.
Hence, to prevent iron articles from getting in contact with moisture paint is applied over them. Paint prevent iron articles from getting destroyed because of the formation of rust over them.
Question: 19: Oil and fat containing food items are flushed with nitrogen. Why?
Answer: Food containing oil and fat when come in the contact with oxygen present in the air, oxidation of fat and oil takes place, because of which food starts giving bad taste and smell, i.e. food becomes rancid. This process of oxidation of oil and fat present in food items is called rancidity.
To prevent food containing oil and fat from getting rancid, nitrogen is flushed with nitrogen.
Question: 20: Explain the following terms with one example each.
(a) Corrosion
(b) Rancidity
Answer:
(a) Corrosion:
Gradual destruction of metals because of formation of respective oxide, hydroxide, or sulphide, etc. because of chemical reaction with substances present in the environment is called corrosion.
Metals react with oxygen, sulphide, moisture, etc. present in the environment. A layer of different colours of metals oxides, sulphides or hydroxide deposited over them. Because of the formation of these layers; metals first lose their lustre and gradually get destroyed.
Rusting of iron, tarnishing of copper and silver, etc. are some common examples of corrosion.
(b) Rancidity:
The spoilage of food items specially made of using oils because of hydrolysis and oxidation of fats present in food is called rancidity.
When a food item is exposed to air, because of oxidation of fats present in food items food gets rancid. When food gets rancid, it gives a bad taste and starts giving bad smells. Rancidity also degrades the nutritional value of food. Eating of rancid food may lead to health problems.