Metals & Non-metals - Class 10th Science
NCERT In Text Solution2
Question : 8. (i) Write the electron-dot structures for sodium, oxygen and magnesium.
(ii) Show the formation of Na2O and MgO by the transfer of electrons.
(iii) What are the ions present in these compounds?
Answer: (i)
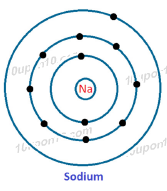
Dot structure for Sodium (11)
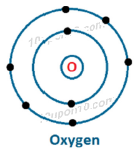
Dot structure of Oxygen (8)
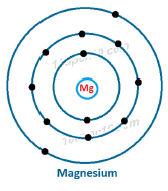
Dot structure of Magnesium (12)
(ii) Formation of Na2
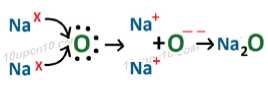
In sodium oxide (Na2O), sodium ion and oxide ion are present.
Formation of MgO
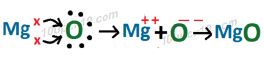
In Magnesium oxide, Magnesium ion and oxide ion are present.
Question: 9. Why do ionic compounds have high melting points?
Answer: Ionic compounds are formed because of ionic bonds formation. Ionic bonds are held together because of electrostatic forces of attraction, these forces of attraction are very strong and it is very difficult to break them. A considerable amount of energy is required to break inter ionic attraction in ionic compounds. This is the cause that ionic compounds have high melting points.
10. Define the following terms.
(i) Mineral (ii) Ore (iii) Gangue
Answer: (i) Mineral: Naturally occurring elements or compounds in earth’s crust are called minerals.
(ii) Ore: Minerals which contain a very high percentage of a particular metal and the metal can be profitably extracted from it are called ores.
(iii) Gangue: Gangue is the impurities present in ores mined from earth. Gangue must be removed from ores before extraction of metal.
11. Name two metals which are found in nature in the free state.
Answer: Silver and Gold. Since silver and gold are least reactive, so these are found in free state.
12. What chemical process is used for obtaining a metal from its oxide?
Answer: Reduction is used for obtaining a metal from its oxide. Reduction is a process in which metal oxide loses oxygen and reduced to respective metal.
Example:
Zinc oxide obtained after the process of roasting or calcinations is reduced to zinc metal. In this process, zinc oxide is heated with a reducing agent, such as carbon to convert zinc oxide into zinc metal.
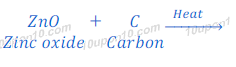
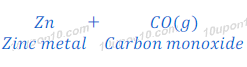
Question: 13. Metallic oxides of zinc, magnesium and copper were heated with the following metals.
| Metal | Zinc | Magnesium | Copper |
|---|---|---|---|
| Zinc oxide | |||
| Magnesium oxide | |||
| Copper oxide |
In which cases will you find displacement reactions taking place?
Answer:
| Metal | Zinc | Magnesium | Copper |
|---|---|---|---|
| Zinc oxide | No reaction | Displacement | No reaction |
| Magnesium oxide | No reaction | No reaction | No reaction |
| Copper oxide | Displacement | Displacement | No reaction |
Explanation:
(i) When zinc oxide is heated with magnesium, magnesium displaces zinc and magnesium oxide is formed.
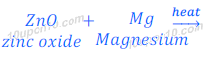
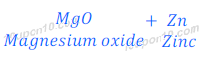
This happens because magnesium is more reactive than zinc.
No reaction takes place when zinc oxide is headed with zinc and copper
(ii) When magnesium oxide is heated with zinc, magnesium or copper, no reaction takes place. Because zinc and copper are less reactive than that of zinc.
(iii) When copper oxide is heated with zinc, zinc displaces copper and zinc oxide is formed. This happens because zinc is more reactive than copper.
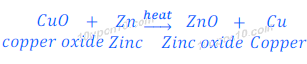
(iv) When copper oxide is heated with magnesium, magnesium displaces copper and forms magnesium oxide. This happens because magnesium is more reactive than copper.
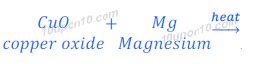
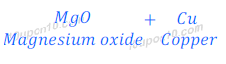
Question: 14. Which metals do not corrode easily?
Answer: Copper, silver and gold do not corrode easily, as these metals are least reactive. These metals are called noble metals.
Question: 15. What are alloys?
Answer:
Alloys are the homogeneous mixture of two or more than two metals or non-metals. Alloying improves the qualities of metal, such as strength, resistance to corrosion, ductility, malleability, etc.
Examples: stainless steel is the alloy of iron, chromium and nickel, bronze is the alloy of copper and tin, brass is the alloy of copper and zinc.