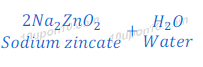Metals & Non-metals - Class 10th Science
NCERT Exercise Solution
Question: 1. Which of the following pairs will give displacement reactions?
(a) NaCl solution and copper metal
(b) MgCl2 solution and aluminium metal
(c) FeSO4 solution and silver metal
(d) AgNO3 solution and copper metal.
Answer: (d) AgNO3 solution and copper metal.
Explanation: Copper metal is more reactive than silver, so copper displaces silver from silver nitrate solution and gives copper nitrate.
Question: 2. Which of the following methods is suitable for preventing an iron frying pan from rusting?
(a) Applying grease
(b) Applying paint
(c) Applying a coating of zinc
(d) All of the above.
Answer: (c) Applying a coating of zinc
Explanation: Applying of grease or paint is not suitable for utensils used for cooking. So, applying of zinc is suitable methods for preventing an iron frying pan from rusting.
Question: 3. An element reacts with oxygen to give a compound with a high melting point. This compound is also soluble in water. The element is likely to be
(a) Calcium
(b) Carbon
(c) Silicon
(d) Iron.
Answer: (a) Calcium
Explanation: Calcium oxide is soluble in water and having high melting point.
Oxides of silicon and iron are not soluble in water. Carbon dioxide is soluble in water but is a gas. Thus, correct option is (a) calciumQuestion: 4. Food cans are coated with tin and not with zinc because
(a) Zinc is costlier than tin.
(b) Zinc has a higher melting point than tin.
(c) Zinc is more reactive than tin.
(d) Zinc is less reactive than tin.
Answer: (c) Zinc is more reactive than tin.
Question: 5. You are given a hammer, a battery, a bulb, wires and a switch.
(a) How could you use them to distinguish between samples of metals and non-metals?
(b) Assess the usefulness of these tests in distinguishing between metals and non-metals.
Answer: A metal is good conductor of electricity and can be hammered into thin sheet. While a non-metal is bad conductor of electricity and ductile, i.e. gets broken while hammered.
(a) Given sample of metals and non-metals first checked using battery, bulb, wires and switch for the conduction of electricity. Samples which conduct electricity are categorized as metals and which do not conduct electricity is categorized as non-metals.
Second, graphite which is a non-metal conducts electricity.
So, samples which conduct electricity are hammered. Those which do not break into pieces are metals and those which are broken into pieces are non-metals.
(b) Since, graphite, which is a non-metal and an allotrope of carbon, also conducts electricity so hammer is used to test for ductility and battery, bulb, wire and switch is meant to test for conduction of electricity.
Thus, both the test is necessary to confirm whether given samples are metals or non-metals.
Question: 6. What are amphoteric oxides? Give two examples of amphoteric oxides.
Answer: Oxides which react as acid with a base and react as base with acid are called amphoteric oxides.
Examples:
Aluminium oxide and zinc oxide are amphoteric oxides.
Reaction of Aluminium Oxide with Hydrochloric Acid
When aluminium oxide reacts with hydrochloric acid, it gives aluminium chloride and water. In this reaction aluminium oxide behaves like a base.


Reaction of Aluminium Oxide with Sodium hydroxide
When aluminium oxide reacts with sodium hydroxide, it gives sodium aluminate and water. In this reaction aluminium oxide behaves like an acid.
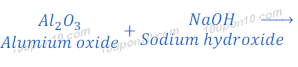
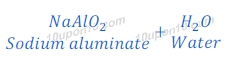
Reaction of Zinc Oxide with Hydrochloric Acid
When zinc oxide reacts with hydrochloric acid, it gives zinc chloride and water. In this reaction zinc oxide acts as a base.

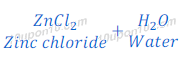
Reaction of Zinc Oxide with Sodium Hydroxide
When zinc oxide reacts with sodium hydroxide, it gives sodium zincate and water. In this reaction zinc oxide acts as an acid.