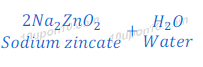Metals & Non-metals - Class 10th Science
Chemical Properties of Metal Oxides
Reaction of Metal oxides with acid
Metal oxides are basic in nature. Thus, when metal oxides react with dilute acid, give respective salt and water.
Metal oxide + Acid → Respective salt + Water
Example:
Reaction of Sodium Oxide with Hydrochloric Acid
(1) When sodium oxide reacts with hydrochloric acid, it gives sodium chloride and water.
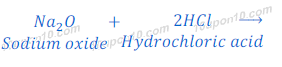
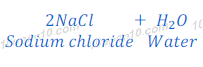
Reaction of Potassium Oxide with Hydrochloric Acid
(2) When potassium oxide reacts with hydrochloric acid, it gives potassium chloride and water.


Reaction of Copper Oxide with Hydrochloric Acid
(3) When copper oxide reacts with hydrochloric acid, it gives copper chloride and water.
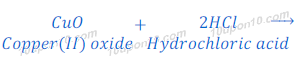
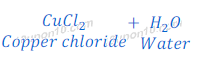
Amphoteric Metal Oxides
Compounds which act as acid when react with a base and act as a base when reacts with an acid are called Amphoteric Compounds.
Where most of the metal oxides are basic, some of the metal oxides are amphoteric in nature. This means some metal oxides react as an acid with a base and react as a base with an acid. For example aluminium oxide and zinc oxide.
Reaction of Aluminium Oxide with Hydrochloric Acid
(1) When aluminium oxide reacts with hydrochloric acid, it gives aluminium chloride and water. In this reaction aluminium oxide behaves like a base.


Reaction of Aluminium Oxide with Sulphuric Acid
(2) When aluminium oxide reacts with sulphuric acid, it gives aluminium sulphate and water.
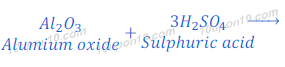
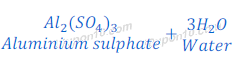
Reaction of Aluminium Oxide with Sodium hydroxide
(3) On the other hand when aluminium oxide reacts with sodium hydroxide, it gives sodium aluminate and water. In this reaction aluminium oxide behaves like an acid.
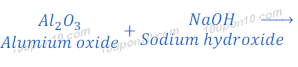
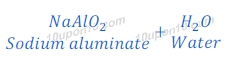
Reaction of Zinc Oxide with Hydrochloric Acid
(4) When zinc oxide reacts with hydrochloric acid, it gives zinc chloride and water. In this reaction zinc oxide acts as a base.

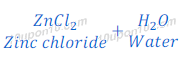
Reaction of Zinc Oxide with Sodium Hydroxide
(5) When zinc oxide reacts with sodium hydroxide, it gives sodium zincate and water. In this reaction zinc oxide acts as an acid.