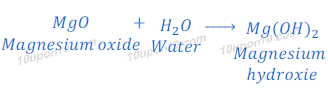Metals & Non-metals - Class 10th Science
Reaction of Metals with water
What happens when Metals react with Water?
Metals form respective metal oxide and hydrogen when react with water.
Metal + Water → Metal Oxide + Hydrogen
Soluble metal oxide gets dissolved in water and form respective metal hydroxide.
Metal oxide + Water → Metal hydroxide
Example:
Reaction of Sodium with water
When sodium metal reacts with water, it gives sodium hydroxide and hydrogen gas. Sodium metal reacts so vigorously with water that hydrogen gas produced in this reaction catches fire immediately.
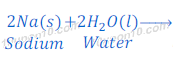
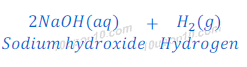
Reaction of sodium with water is highly exothermic.
Reaction of potassium with water
Potassium metal reacts vigorously with water and produces potassium hydroxide and hydrogen.
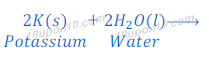
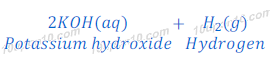
Reaction of potassium and water is highly exothermic. In this reaction also evolved hydrogen gas catches fire immediately.
Reaction of Calcium with water
Calcium metal reacts with water vigorously and produces calcium hydroxide and hydrogen gas.
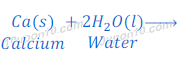
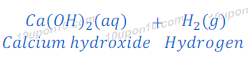
In this reaction of calcium and water, bubbles of hydrogen gas are formed and get stuck to the surface of calcium metal because of which calcium starts floating.
Reaction of calcium with water is exothermic but heat produced in this reaction is not sufficient so that hydrogen can catch fire.
Reaction of Magnesium with water
Magnesium metal does not react with cold water. Magnesium reacts with hot water and forms magnesium hydroxide and hydrogen gas.
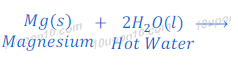
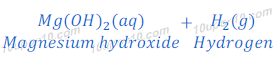
In this reaction of magnesium and water, magnesium starts floating because of bubbles of evolved hydrogen gas get stuck with the surface of magnesium metal.
Reaction of Aluminium metal with water
When aluminium metal reacts with steam, alunimium oxide and hydrogen gas are formed.
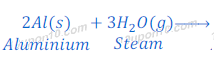
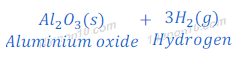
Aluminium does not react with cold or hot water. But aluminium reacts with steam.
Reaction of zinc with water
When zinc reacts with steam, zinc oxide and hydrogen gas are formed.
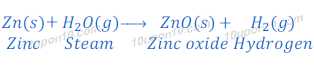
Zinc also do not react with cold or hot water.
Reaction of Iron with water
Iron also does not react with cold or hot water, but when reacts with steam, iron oxide and hydrogen gas are formed.
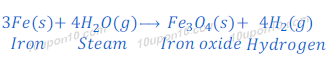
Rusting of Iron
Iron reacts with cold water or moisture present in atmosphere very slowly and iron oxide is formed in the form of layer over iron. Iron oxide is red in colour. This process of formation of iron oxide in the form of flakes over the surface of iron is called rusting of iron.
Reaction of lead, copper, silver and gold with water
Metals such as lead, copper, silver and gold do not react either with cold water, hot water or steam at all.
Reaction of Metal Oxides with Water
Most of the metal oxides are insoluble in water, but oxides of alkali metals and alkaline earth metals are soluble in water. Water soluble metal oxides react with water and gives, respective base. These bases are very strong and are called alkali.
Example:
Reaction of Sodium oxide with water
When sodium oxide reacts or dissolves in water, it gives sodium hydroxide.
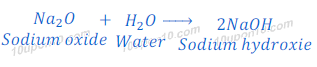
Sodium hydroxide is a strong base.
Reaction of potassium oxide with water
When potassium oxide reacts with water or dissolves in water, it gives potassium hydroxide.
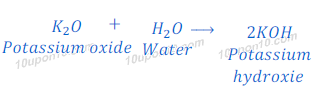
Reaction of magnesium oxide with water
When magnesium oxide is dissolved in water, it gives magnesium hydroxide.