Metals & Non-metals - Class 10th Science
Ionic Bond or Electrovalent Bond
Chemical bond formed after transfer of electrons by two atoms is called Ionic bond or electrovalent bond. In other words, since such type of bond is formed by ions so it is called IONIC BOND. Or this type of bond is formed by electrically charged particles so it is called ELECTROVALENT BOND.
When a metal reacts with a non-metal, bond formed by them is called Ionic Bond or Electrovalent bond. After reaction atoms of metal and non metal get bonded together by electrostatic force of attraction which is called chemical bond.
Example
Formation of NaCl (Sodium Chloride)
Atomic number of Na (Sodium) which is a metal = 11
Electronic configuration of Na (Sodium) = 2, 8, 1
Valence electron in sodium atom = 1
Atomic number of Chlorine (Cl) which is a non-metal = 17
Electronic configuration of chlorine = 2, 8, 7
Valence electrons of chlorine = 7
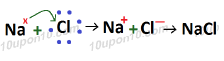
Sodium metal has tendency to acquire 8 electrons in order to get stable configuration. And sodium has one electron in its outermost orbit which it can lose easily. So, sodium has always tendency to lose one electron from its outermost orbit.
Whereas chlorine has tendency to acquire 8 electrons in its outermost orbit in order to get stable configuration, i.e. chlorine has tendency to gain one electron.
Thus, in formation of sodium chloride, sodium loses one electron and transfers it to chlorine atom. And chlorine gains one electron loses by sodium atom.
After losing one electron, sodium gets one positive charge over it. And after gains of one electron chlorine gets one negative charge over it. After combination of one sodium ion (Na +) and chloride ion (Cl–), sodium chloride is formed which become neutral by the combination of one positive and one negative charge.
Bond formed in NaCl is called Ionic Bond or electrovalent bond, and compound formed because of ionic bond is called Ionic compound or electrovalent compound.
Formation of KCl (Potassium Chloride)
Atomic number of K (Potassium) which is a metal = 19
Electronic configuration of K (Potassium) = 2, 8, 8, 1
Valence electron in potassium atom = 1
Atomic number of Chlorine (Cl) which is a non-metal = 17
Electronic configuration of chlorine = 2, 8, 7
Valence electrons of chlorine = 7
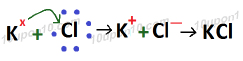
Bond formed in KCl (Potassium chloride) is called Ionic Bond or electrovalent bond, and compound formed because of ionic bond is called Ionic compound or electrovalent compound.
Formation of MgCl2 (Magnesium chloride)
Atomic number of Mg (Magnesium) which is a metal = 12
Electronic configuration of Mg (Magnesium) = 2, 8, 2
Valence electron in magnesium atom = 2
Atomic number of Chlorine (Cl) which is a non-metal = 17
Electronic configuration of chlorine = 2, 8, 7
Valence electrons of chlorine = 7
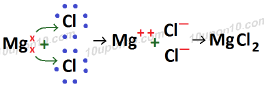
Bond formed in the formation of magnesium chloride is called ionic bond or electrovalent bond and compound so formed is called ionic compound or electrovalent compound.
Formation of calcium chloride (CaCl2)
Atomic number of Ca (Calcium) which is a metal = 20
Electronic configuration of Ca (Calcium) = 2, 8, 8, 2
Valence electron in calcium atom = 2
Atomic number of Chlorine (Cl) which is a non-metal = 17
Electronic configuration of chlorine = 2, 8, 7
Valence electrons of chlorine = 7
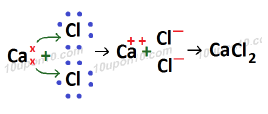
Since bond formed in calcium chloride is because of transfer of electrons, thus bond in calcium chloride is called ionic bond or electrovalent bond and calcium chloride is called ionic or electrovalent compound.
Formation of Calcium bromide (CaBr2)
Atomic number of Ca (Calcium) which is a metal = 20
Electronic configuration of Ca (Calcium) = 2, 8, 8, 2
Valence electron in calcium atom = 2
Atomic number of Bromine (Br) which is a non-metal = 35
Electronic configuration of bromine = 2, 8, 18, 7
Valence electrons of bromine = 7
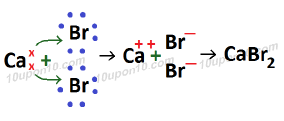
Calcium bromide is called ionic compound or electrovalent compound. And bond formed in calcium bromide is called ionic bond or electrovalent bond.
Formation of Magnesium Bromide (MgBr2)
Atomic number of Mg (Magnesium) which is a metal = 12
Electronic configuration of Mg (Magnesium) = 2, 8, 2
Valence electron in magnesium atom = 2
Atomic number of Bromine (Br) which is a non-metal = 35
Electronic configuration of bromine = 2, 8, 18, 7
Valence electrons of bromine = 7
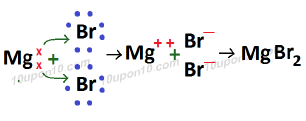
Bond present in magnesium bromide is called ionic or electrovalent bond. And magnesium bromide is called ionic or electrovalent compound. Because this compound is formed after transfer of electrons.
Formation of Magnesium fluoride (MgF2)
Atomic number of Mg (Magnesium) which is a metal = 12
Electronic configuration of Mg (Magnesium) = 2, 8, 2
Valence electron in magnesium atom = 2
Atomic number of Fluorine (F) which is a non-metal = 9
Electronic configuration of fluorine = 2, 7
Valence electrons of fluorine = 7
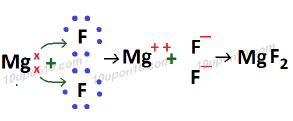
Similarly, magnesium fluoride is called ionic or electrovalent compound. And bond formed in this is called ionic or electrovalent bond.
Properties of Ionic Compounds
(1) Physical nature of Ionic compounds
(a) Ionic compounds are solid
(b) Ionic compounds are hard upto some extent. Hardness of ionic compound is because of strong electrostatic force of attraction between negative and positive ions.
(c) Ionic compounds are brittle in nature. Ionic compounds get broken if pressure is applied over it.
(2) Melting and Boiling points of Ionic compounds
Ionic compounds have high melting and boiling point. Since ionic bond are very strong, hence to break them more energy is required, this makes boiling and melting point of ionic compounds higher.
(3) Solubility of Ionic compounds
Ionic compounds are generally soluble in water. Since ionic compounds are formed because of ions, so these compounds are soluble in water. But Ionic compounds are insoluble in kerosene, petrol, etc.
(4) Conduction of electricity of Ionic compounds
(a) Ionic compounds conduct electricity in its molten state or in solution.
When ionic compounds are dissolved in water, their ionic components breaks into ions which are responsible for conduction of electricity. In molten state also movable charged particles of ionic compounds conduct electricity.
(b) Ionic compounds do not conduct electricity in their solid state.