Metals & Non-metals - Class 10th Science
Occurrence of Metal
Metal are found in earth's crust. Metals are also found in seawater in the form of many soluble salts. For example sodium chloride, magnesium chloride, etc. are salts which are found dissolved in sea water. From these salts metals like sodium, magnesium, etc. are extracted.
Minerals
Elements or compounds which occur naturally in earth crust are called MINERALS.
Ores
Minerals from which metals can be extracted profitably are called ores.
Extraction of Metals
Metals are extracted from their ores on the basis of reactivity series. To extract them from ores, metals are divided into following three categories.
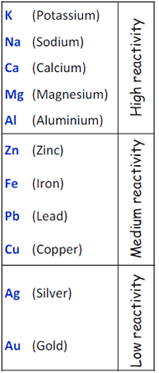
Metals in the bottom of Reactivity Series
Metals found at the bottom of reactivity series are very less reactive or non-reactive. These metals found in free state in the earth's crust.
Example: Silver and Gold are at the bottom of reactivity series. So silver and gold are found in earth's crust in free state.
Copper and silver are also found in combined states as in the form of their oxides and sulphide ores.
Metals in the middle of Reactivity Series
Metals found in the middle of reactivity series are moderately reactive, so they are mainly found in the form of their oxides, sulphides or carbonate. Zinc, Iron, lead and copper fall in the middle of the reactivity series.
Metals in the top of Reactivity Series
Metals lies in the top of the reactivity series are very much reactive. They never find in nature in free state. Potassium, sodium, calcium, magnesium and aluminium fall in the top of the reactivity series.
Metallurgy
Process of extraction of pure metal from its ore is called Metallurgy.
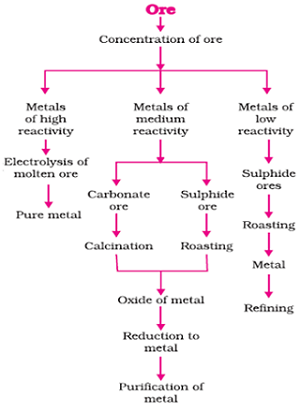
Steps involved in extraction of pure metal from its ores.
Concentration of Ores
Gaunge: Ores mined usually contained many impurities , such as soil, sand, etc. This impurities of ores is called Gaunge.
First impurities removed from gaunge. Removing of impurities from Gaunge is called Concentration of Ores.
Many types of processes are applied to remove impurities from Gaunge. These processes to separate impurities from ores depend upon the physical and chemical properties of Gaunge and ore.
Concentration of ores is known as enrichment of ores also.
Roasting
Heating of ores of metals found in the form of sulphide in the presence of air is called the process of Roasting. Roasting converts ores in the form of sulphide to the respective oxides.
Calcination
Heating of ores of metals found in the form of carbonate in the presence of limited air is called the process of Calcination. Calcination of carbonate ores converts them into respective oxides.
Reduction
The process of converting metal oxides to respective metals by heating is called Reduction. Reduction converts oxides of metals into respective metals.
What is the need of Roasting or Calcination?
Since it is easier to obtain a metal from its oxide compare to respective sulphides or carbonates, so roasting or calcinations is needed to convert ores of metals found in the form of sulphides or carbonates into their respective oxides.