Carbon and Its Compounds - Class 10th Science
Ehtanol & Ethanoic Acid
Some Important Carbon Compounds - Ethanol and Ethanoic Acid
Ethanol and Ethanoic Acid are some of the most important carbon compounds.
Ethanol
The common name of Ethanol is Ethyl Alcohol. It is generally called as simply Alcohol because it is the active ingredient of all alcoholic drinks.
Consuming of a small quantity of dilute ethyl alcohol causes drunkenness, however taking of even a small quantity of pure alcohol may prove lethal.
Pure alcohol is known as absolute alcohol.
Physical Properties of Ethanol:
Ethanol is a volatile and colourless liquid.
Ethanol is liquid at room temperature.
Ehtanol has a pleasant smell.
Melting point of Ethanol is – 1140C and boiling point is 78.370C.
Ethanol is soluble in water in all proportions.
Ethyl alcohol (Ethanol) is very good solvent. Ethanol is used in many medicines, such as tincture iodine, cough syrups, etc.
Chemical properties of Ethanol
Reaction of Ethanol with sodium
Ethanol gives sodium ethoxide when reacts with sodium along with hydrogen gas.
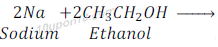
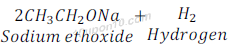
Reaction to give Unsaturated Hydrocarbon
Ethanol gives ethylene (Ethene) when reacts with Hot concentrated H2SO4 along with water. Concentrated sulphuric acid can be regarded as dehydrating agent as it removes water from Ethanol.
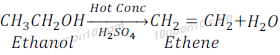
Ethanoic Acid
Ethanoic acid is another one of the most important produces of carbon. The common name of ethanoic acid is Acetic acid.
The 5% – 8% solution of acetic acid in water is called Vinegar. Vinegar is used as food preservative in pickles.
The melting point of ethanoic acid is 16.850C [290K].
Acetic acid often freezes during winter season in cold climate and thus it is called glacial acetic acid also.
Similar to all other organic acids, acetic acid is also a weak acid.
Reactions of Ethanoic Acid
Esterification Reaction
Esters are formed most commonly after the reaction of an acid and alchohol. Esters have sweet fragrance and are used in making of perfumes and used as flavoring agent.
When ethanoic acid reacts with ethanol in the presence of an acid as a catalyst, it gives ehthy acetate (ester).
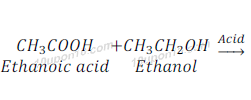
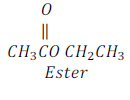
This is called Esterification or Esterification Reaction.
Saponification:
On the other hand when ester is treated with sodium hydroxide, it gives alcohol and sodium salt of carboxylic acid. This reaction is called saponification as it is used in manufacturing of soap.
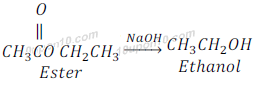
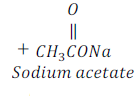
Sodium acetate is called sodium ethanoate also.
Reaction of Ethanoic acid with a base
When ethanoic acid reacts with sodium hydroxide, which is a base, it gives sodium acetate and water.
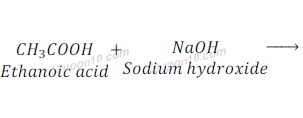
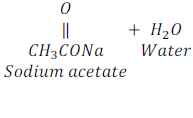
Reaction of Ethanoic Acid with Carbonates and Hydrogen carbonates
Ethanoic acid gives respective acetate, carbon dioxide and water.
When Ethanoic acid reacts with sodium carbonate, it gives sodium acetate, water and carbon dioxide.
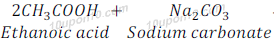
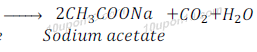
When Ethanoic acid reacts with sodium hydrogen carbonate, it gives sodium acetate, water and carbon dioxide.
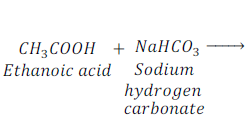
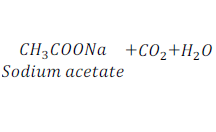
Soaps and Detergents
Soaps are salts of long chain carboxylic acids. While detergents are generally ammonium or sulphonate salts of long chain carboxylic acids.
Cleansing of cloth using soap or detergent
When soap is mixed in water, it forms a structure called micelles. Because of formation of micelles we see the foam of soap in water.
In micelles one end of soap molecule is towards the oil while the ionic end faces outside. This forms an emulsion in water.
Dirt in clothes contains oil which gets stuck with clothes. When cloth is dip in soap water, soap micelle helps in dissolving dirt present in cloths and water which can be washed out while rinsing, this cleans the cloths.
Micelles
Soap molecules have two ends, one end is ionic end and other one is carbon chain. Ionic end dissolves in water while carbon chain dissolves in oil. Ionic end which dissolves in water is called hydrophilic and carbon chain is hydrophobic.
When soap is at the surface of water, the hydrophobic 'tail' which is not soluble in water aligns along the surface of water with the ionic end in water and hydrocarbon 'tail' protruding out of water. Inside water, these molecules have unique orientation that keeps hydrocarbon portion outside of water.
This is achieved by forming clusters of molecules in which hydrophobic tails are in the interior of the cluster and the ionic ends are on the surface of cluster. This formation is called micelle.
Soap in the formation of micelles traps dirt in the centre. The micelles stay in the solution as colloid because of ion-ion repulsion do not come together to precipitate. Thus, dirt suspended in micelles easily rinsed away.
The soap micelles are large enough to scatter light. Hence a soap solution appears cloudy.
Formation of Scum
Calcium and magnesium salt dissolved in water are cause of hardness of water. Soap does not form enough lather or even does not form lather at all in hard water. This happens because of soap molecules react with presence of calcium and magnesium salt present in water and form insoluble precipitate. This insoluble precipitate is called Scum.
It is seen that after bathing with soap, a white type substances remain present on the body even after bathing. This white substance is scum. Scum can be washed out easily and to wash out scum a larger amount of soap and more water is needed.
Detergent
Detergents are generally ammonium or sulphonate salts of long chain carboxylic acids. Detergents clean clothes even in hard water.
When detergent is mixed in water, the charged ends of detergent molecules do not form insoluble precipitates with the calcium and magnesium ions present in hard water and clean clothes in better way. Because of these properties detergent is preferred at the place of soap for cleaning.
Detergents are used to make shampoos and products for cleaning clothes.