Carbon and Its Compounds - Class 10th Science
NCERT Intext Solutions
Question: 1. What would be the electron dot structure of carbon dioxide which has the formula CO2?
Answer: Electron dot structure of carbon dioxide (CO2)
Atomic number of carbon (C) = 6
Electronic configuration of carbon (C) = 2, 4
Valence electrons of carbon (C) = 4
Atomic number of oxygen (O) = 8
Electronic configuration of oxygen (O) = 2, 6
Valence electrons of oxygen (O) = 6
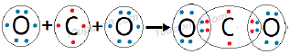
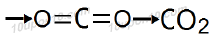
In the formation of molecule of carbon dioxide, carbon shares two electrons with each of the two oxygen atoms while each of the two oxygen atoms share two electrons with carbon atom in order to achieve stable configuration.
Bonds present in carbon dioxide are covalent bonds and carbon dioxide is called covalent compound.
Question: 2. What would be the electron dot structure of a molecule of sulphur which is made up of eight atoms of sulphur? [Hint: The eight atoms of sulphur are joined together in the form of a ring.]
Answer: Electron dot structure of sulphur:
Atomic number of sulphur (S) = 16
Electronic configuration of sulphur (S) = 2, 8, 6
Valence electron of sulphur (S) = 6
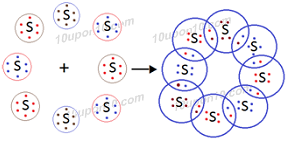
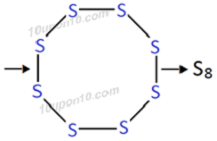
The molecular formula of sulphur is S8
Question: 3. How many structural isomers can you draw for pentene?
Answer: There are three structural isomers can be drawn for pentane.
These are normal pentane (or n–pentane), iso pentane and neo pentane.
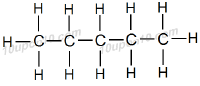
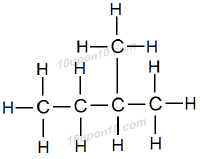
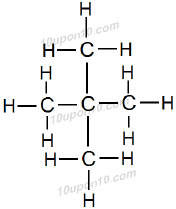
Question: 4. What are the two properties of carbon which lead to the huge number of carbon compounds we see around us?
Answer: Tetra valency and catenation are two properties of carbon which leads to the formation of huge number of carbon compounds.
Tetravalency: Because of tetra valency carbon can make bonds with four carbon atoms, or with four atoms of other elements.
Catenation: The linkage of atoms of same elements to form longer chain is called the CATENATION.
Because of this property of catenation, carbon can linked with other carbon atoms forming single bond, double or triple bonds between them forming straight chain, side chain or chain in ring form.
Thus, these two properties of carbon lead to the formation of a huge number of carbon compounds.
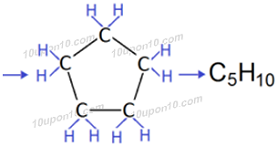
Question: 5. What will be the formula and electron dot structure of cyclopentane?
Answer: The molecular formula of cyclopentane is C5H10
Electron dot structure of cyclopentane
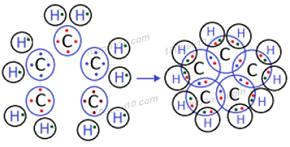
Question: 6. Draw the structures of the following compounds.
(i) Ethanoic acid
(ii) Bromopentane
(iii) Butanone
(iv) Hexanal
(v) Are the structural isomers possible for bromopentane?
Answer:
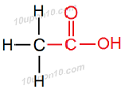
(i) Ethanoic Acid
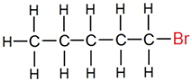
(ii) Bromopentane
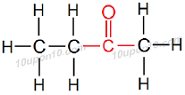
(iii) Butanone
(iv) Hexanal
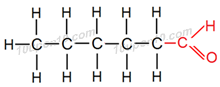
(v) Yes, structural isomers are possible for bromopentane.
Structural formula of some of the structural isomers of bromopentane.
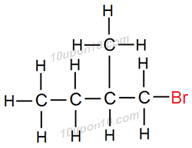
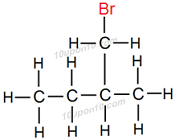
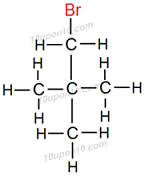
Carbon compounds having same molecular formula but different structural formula are called structural isomers. Above all compounds have same molecular formula i.e. C5H11Br but different structural formulas.
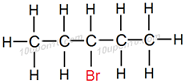
Question: 7. How would you name the following compounds?
(i)CH3–CH2–Br
(ii) 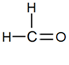
(iii) 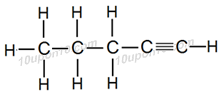
Answer: (i) Bromoethane or Ethyl bromide
Explanation:
This compound has two carbon atoms thus, name of parent compound is ethane and bromide (–Br) is attached as functional group, thus name of this compound is bromoethane. General name of this compound is Ethyl bromide.
(ii) Methanal or Formaldehyde
Explanation:
This compound has one carbon atom and an aldehyde (–CHO) is attached with it. Thus, IUPA name of this compound is methanol. The general name of this compound is formaldehyde.
(iii) Pentyne
Explanation:
There are five carbon atoms and one triple bond is present in this compound, thus IUPAC name of this compound is Pentyne.
Question:8. Why is the conversion of ethanol to ethanoic acid an oxidation reaction?
Answer: Addition of oxygen or replacement of hydrogen from a compound during a chemical reaction is called oxidation reaction. Since, in the conversion of ethanol to ethanoic acid, oxygen is added to ethanol after replacement of hydrogen to form ethanoic acid, thus, this reaction is called oxidation reaction.
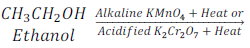
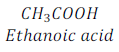
Question: 9. A mixture of oxygen and ethyne is burnt for welding. Can you tell why a mixture of ethyne and air is not used?
Answer: Air is a mixture of many gases carbon dioxide is one of them. When ethyne is allowed to burn with air, gases other than oxygen present in air prevent ethyne from complete combustion and high temperature required for welding is not achieved. But while ethyne is burnt with only oxygen ethyne is burnt with clean flame and complete combustion takes place along and it gives a high temperature of 30000C which gives proper welding.
This is the cause that ethyne is burnt with only oxygen and not with the air for welding.
Question: 10. How would you distinguish experimentally between an alcohol and a carboxylic acid?
Answer: Acid gives carbon dioxide when reacts with carbonates while alcohol does not. Carbon dioxide turns lime water milky.
Thus, by reaction with carbonates or hydrogen carbonates and gas evolved is passed through lime water, alcohol and carboxylic acid can be distinguished experimentally.
Question: 11. What are oxidizing agents?
Answer: Substances which gives oxygen or removes hydrogen from a substance while chemical reaction is called oxidizing agents.
Example:
When Zinc oxide reacts with carbon, it gives zinc and carbon monoxide.
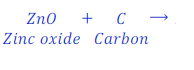
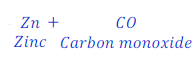
Here,
ZnO gives oxygen to hydrogen and hence is a oxidizing agent.
Question: 12. Would you be able to check if water is hard by using a detergent?
Answer: Soap does not form or form very less lather with hard water while forms lather with soft water. But detergent forms lather with hard and soft water both.
Thus, it is not possible to check if water is hard by using a detergent.
Question: 13. People use a variety of methods to wash clothes. Usually after adding the soap, the beat the clothes on stone, or beat it with paddle, scrub with a brush or the mixture is agitated in a washing machine. Why is agitation necessary to get clean clothes?
Answer: Soap forms micelles which trap dirt and clothe is get cleaned by rinsing these micelles out. Thus, agitation is necessary to get all dirt particles trapped completely by micelles which can be rinsed out after to clean clothes properly.